 |
 |
AbstractBackground and Objectives Fungal biofilm is commonly found in non-invasive fungal rhinosinusitis. Previous endodontic maxillary teeth treatments have increased fungus ball development in the maxillary sinus. We sought to evaluate the effects of zinc oxide (ZnO), the main component of endodontic sealers, on developing the Aspergillus fumigatus; biofilms on primary human nasal epithelial cells.
Subjects and Method Primary human nasal epithelial cells were cultured with A. fumigatus; spores with (1 and 3 µg/mL) or without ZnO for 72 h. Interleukin (IL)-6, IL-8, and transforming growth factor (TGF)-β1 levels in cultural supernatant were determined by enzyme linked immunosorbent assay. ZnO’s effects on the formation of A. fumigatus biofilm were determined using crystal violet, safranin, concanavalin A staining, and confocal scanning laser microscopy.
Results IL-6, IL-8, and TGF-β1 protein levels in primary human nasal epithelial cells increased significantly by A. fumigatus exposure. During coculturing with ZnO and A. fumigatus, biofilm dry weight, crystal violet, safranin, and concanavalin A staining intensity increased with time. On the other hand, ZnO did not enhance A. fumigatus biofilm formation.
IntroductionFungi are environmentally ubiquitous as saprophytes and usually coexist peacefully without harmful effects to the host. Fungus ball (FB) is the most common non-invasive fungal rhinosinusitis. It is characterized by accumulated fungal hyphae within the sinus cavity but with no microscopic invasion into sinonasal tissues. FB prevalence has progressively increased over the last 10 years [1,2]. FB incidence has increased due to the increased use of antibiotics, global warming, and dental procedures. The improved quality of diagnostic imaging technique can be found FB incidentally during the medical health checkups or neurologic evaluation of intracranial pathologies [3].
Aspergillus species are the most commonly reported cause of fungal disease, and Aspergillus fumigatus is the main cause of sinus FB [4]. FB within the lung is morphologically characterized as hyphae in a complex multicellular structure, biofilmlike structure and is resistance to antifungal treatment [5]. Bacterial coinfections, such as coagulase-negative Staphylococcus, Staphylococcus aureus, and Enterobacter aerogenes are commonly found in sinus secretion of FB [6]. Bacterial and fungal biofilm commonly coexisted in the FB sinus [7]. While A. fumigatus dose not form biofilms on the sinus mucosa, under particular conditions such as damaged ciliary clearance or appropriate substrates for fungal growth, the formation of fungal biofilms may be induced.
In most cases, FB is in the maxillary sinus, however, FB pathogenesis and risk factors remain largely unknown. Previous endodontic maxillary teeth treatment and grafting of the maxillary sinus floor for dental implant placement, with deproteinized bovine bone substitutes, increase FB development in the maxillary sinus [8,9]. Upper molar and premolar teeth are close to the maxillary sinus floor. Root canal treatment with root canal filler or zinc oxide (ZnO) endodontic sealers can enter the maxillary sinus to develop FB in the maxillary sinus [8-10]. ZnO is an essential microelement that facilitates fungal survival, growth, and proliferation [10,11]. ZnO paralyzes ciliary movement of the sinonasal epithelium or induces mucosal edema and hyperemia, thereby impairing the elimination of fungal spores and accumulated fungal elements, calcium phosphate, and calcium sulfate [11]. Authors studied the effect of ZnO on A. fumigatus biofilm formation on primary human nasal epithelial cells in this research.
Subjects and MethodPreparation of A. fumigatus sporesSupplier of A. fumigatus was one of professor affiliated to department of Clinical Pathology of author’s medical center. Fungal conidia were isolated according to a previously described method, with minor modification [12]. To collect the conidia, the plate surface was washed with 5 mL of phosphate buffer saline (PBS) added with 0.05% Tween 20. Centrifugation of eluates was performed at 1000 rpm for 5 min and pellet suspensions filtered through a 40 μm cell-strainer. 2×107/mL conidia suspensions were dried at 45°C and stored at -80°C until needed.
Coculturing primary human nasal epithelial cells and A. fumigatusPrimary human nasal epithelial cells were collected from the inferior turbinate of 12 patients who have deviated septum (five female and seven male; aged 45.3±15.2 years) during septal surgery [13]. Subjects who had allergy, active inflammation, and used antihistamines, antibiotics, or other medication for at least four weeks preoperativelywere excluded. The multiple allergen simultaneous test and skin prick test were used to evaluate patients’ allergy status. This study was approved by the Institutional Review Board of the medical institution to which the authors’ belongs (CR-1 9-102), and all participants signed informed consent explaining the purpose of the study.
Specimens were placed in Ham’s F-12 medium added with 2 µg/mL amphotericin B, 100 µg/mL streptomycin, 100 IU penicillin. The nasal mucosa was rinsed in this media and incubated with 0.1% dispase (Roche Diagnostics, Mannheim, Germany) at 4°C for 16 h. Epithelial cells were isolated by agitation gently, and no. 60 mesh cell dissociation sieve was used to filter the cell suspensions. Then, cells were suspended in RPMI-1640 medium which was supplemented with 5 µg/mL transferrin, 150 µg/mL glutamine, 15 µg/mL endothelial cell growth supplement, 25 ng/mL epithelial growth factor, 5 IU/mL insulin, 200 pM triiodothyronine, 15% fetal calf serum and 100 nM hydrocortisone. Cell suspensions (106 cells/mL) were then incubated at 37°C with 5% CO2. When primary human nasal epithelial cells reached 80%-90% confluence, they were treated with A. fumigatus (1×105/mL) for 2 h, after which were washed to get rid of the non-adherent fungus. Then, cells were cultured in media for 72 h, including 1 µg/mL or 3 µg/mL ZnO.
Cell proliferation assayZnO’s cytotoxic effects were evaluated with CellTiter-96® aqueous cell proliferation assay kit (Promega, Madison, WI, USA). Primary human nasal epithelial cells were cultured with ZnO at various concentrations (0.1, 1, 3, and 10 µg/mL) for 72 h at 37°C with 5% CO2 in a 96-well microstate plate. After this period, Owen’s reagent and tetrazolium compound were added to each well. The reduced tetrazolium compound produced a colored formazan product due to intracellular mitochondrial activity. The generated formazan was directly proportional to viable cell numbers. Absorbance was evaluated using a fluorescence microplate reader at 490 nm.
Detection of interleukin (IL)-6, IL-8, and transforming growth factor (TGF)-β1When ZnO and A. fumigatus was co cultured during 24 h, 48 h, 72 h, cells and supernatant were harvested and then stored at -70°C for assay. We measured immunoreactive IL-6, IL-8, and TGF-β1 protein levels in supernatants with enzyme-linked immunosorbent assay kits (R&D system, Minneapolis, MN, USA) by following the manufacturer’s instructions.
Biofilm dry weightWe collected culture materials by scraping and filtering through a cellulose nitrate filter (pore size with 0.45 μm; Sartorius, Göttingen, Germany) to quantify the biofilm dry weight [12]. They were dried to a constant weight at 40°C.
Biofilm quantification
A. fumigatus biofilm was quantified using Mowat method [14]. 0.5% crystal violet solution was added at 96 well culture plate for 5 minutes, cocultured primary human nasal epithelial cells and A. fumigatus. The plate was washed with PBS and biofilms were unstainedfor 1 minute by adding 100 μL of 95% ethanol to each well. The ethanol was transferred to another microtiter plate and the optical density was measured at 570 nm with spectrophotometer (FLUOstar OPTIMA; BMG LABTECH, Oldenburg, Germany).
Detection of an extracellular matrix of fungal biofilmWe stained the coculture plate, primary human nasal epithelial cells, and A fumigatus with 50 μL of safranin solution after 24 h, 48 h, and 72 h incubation to detect the polysaccharide structure of fungal biofilm. After 5 minutes, the plate was washed with PBS and spectrophotometer (BMG LABTECH) was used to measure the optical density at wave length of 492 nm.
Concanavalin A binds to the fungal cell wall’s extracellular matrix. We stained the culture plate in 100 μL of 25 μg/mL Alexa Fluor 488 (Invitrogen, Carlsbad, CA, USA) conjugated to succinylated concanavalin A for 45 min at 37°C. Three times washing was done in plate by PBS. To measuring the fluorescence intensity, we used a spectrophotometer (BMG LABTECH) at excitation and emission wavelengths of 485 nm and 520 nm respectively.
Biofilm confocal scanning laser microscopyWe stained the coculture plate at 24 h, 48 h, and 72 h. The culture plates were washed with PBS and incubated in dark for 45 min with 300 μL of PBS containing 100 μM of FUN-1 (Invitrogen) and 100 μg/mL of concanavalin A. Excess stain was removed by washing in PBS. Nikon A1 confocal microscope (Nikon, Tokyo, Japan) was used to determine image capture and analyses. Green fluorescence area, concanavalin A, represented cell wall-like polysaccharides, and red fluorescence, FUN-1, indicated metabolically active cells, while yellow areas represented dual staining.
Statistical analysisAll experimental procedures were repeated at least five times with similar results. The results are presented as mean ±standard deviation. To evaluate the difference between two groups, Student’s t-test was performed and data among several groups were analyzed by one-way analysis of variance followed by a Tukey’s test (SPSS ver. 25.0; IBM Corp., Armonk, NY, USA). p-values below 0.05 were considered statistically significant.
ResultsCytotoxic effect of ZnO on primary human nasal epithelial cellsPrimary human nasal epithelial cells were treated with several concentrations of ZnO for 72 h to determine the optimal concentration for further studies. Cell viability decreased significantly at 10 μg/mL of ZnO over 24 h incubation. Therefore, we used under 3 μg/mL of ZnO for further experiments (Fig. 1).
Production of chemical mediators by A. fumigatusTGF-β1, IL-6, IL-8 production from primary human nasal epithelial cells is induced by Aspergillus. When the primary human nasal epithelial cells collected from A. fumigatus and ZnO for 48 h and 72 h, IL-8 production was significantly decreased. However, Aspergillus-induced IL-6 and TGF-β1 production was not influenced by ZnO (Fig. 2).
Effects of ZnO on the A. fumigatus biofilm formation
A. fumigatus biofilm dry weight was determined by measuring scraped extracellular matrix after 24 h, 48 h, and 72 h. When A. fumigatus was culture on primary human nasal epithelial cells, the dry weight of biofilm was 1.9±0.2 mg after 24 h, 2.7±0.3 mg after 48 h, and 3.0±0.1 mg after 72 h. Biofilm dry weight was significantly increased by culturing A. fumigatus on primary human nasal epithelial cells or coculturing A. fumigatus with ZnO on primary human nasal epithelial cells in compare to A. fumigatus which was cultured without primary human nasal epithelial cells. 1 µg/mL and 3 µg/mL of ZnO without primary human nasal epithelial cells did not increase the A. fumigatus biofilm dry weight (Fig. 3A).
To determine biofilm formation, crystal violet staining was used. The optical density of crystal violet was significantly enhanced in coculture of primary human nasal epithelial cells with A. fumigatus compared with A. fumigatus cultured without primary human nasal epithelial cells or only with ZnO. Coculturing of 1 µg/mL and 3 µg/mL of ZnO with A. fumigatus on primary human nasal epithelial cells did not enhance the formation of biofilm (Fig. 3B).
The optical density of safranin which stain the polysaccharide of fungal cell wall increased with time. When A. fumigatus cultured on primary human nasal epithelial cells, the optical density was significantly increased compare with A. fumigatus which was cultured without primary human nasal epithelial cells or only with ZnO. Coculturing of 1 µg/mL and 3 µg/mL of ZnO with A. fumigatus on primary human nasal epithelial cells did not influence the polysaccharide expression of the fungal cell wall (Fig. 3C).
The amount of extracellular matrix produced by A. fumigatus by staining with concanavalin A-Alex Fluor increased with time. The optical density of concanavalin-A was significantly enhanced in coculture of primary human nasal epithelial cells with A. fumigatus compared with A. fumigatus cultured without primary human nasal epithelial cells or only with ZnO. Coculturing of 1 µg/mL and 3 µg/mL of ZnO with A. fumigatus on primary human nasal epithelial cells did not influence the amount of extracellular matrix (Fig. 3D).
Confocal scanning laser microscopic findings of A. fumigatus biofilmConcanavalin A stains the polysaccharides of the extracellular matrix, conidia, hyphae, and free-floating fungi, which produces intense green fluorescence. The green fluorescence intensity by extracellular matrix produced from A. fumigatus increased with time. However, ZnO did not influence concanavalin A intensity of A. fumigatus (Fig. 4D). The red fluorescence intensity by metabolically active A. fumigatus components increased with time. Coculturing of A. fumigatus with 1 µg/mL and 3 µg/mL of ZnO on primary human nasal epithelial cells did not influence FUN-1 fluorescence intensity (Fig. 4E).
Discussion
A. fumigatus is a ubiquitous saprophytic fungus that is the most common species causing FB in sinuses. A. fumigatus conidia contain sialic acid residues and facilitates binding to basal lamina protein of respiratory epithelial cells [15]. ZnO is the main component of endodontic sealers, and zinc has been commonly studied as an essential microelement for fungal adhesion, proliferation, and growth [8,10]. Sinus FB has morphological characteristics of fungal biofilms, and fungal biofilm and bacterial biofilm coexist in sinonasal mucosa of FB [7,16]. We tried to confirm the clinical significance of ZnO for the development of fungal biofilm. However, this in-vitro study demonstrated that ZnO did not significantly affect the development A. fumigatus biofilm.
A. fumigatus accounts for about 0.3% of the fungi present in the air and several hundred conidia enter into the airway by inhalation. Because the conidia size of A. fumigatus is 2.0-3.5 µm, they easily bypass the nasal mucociliary clearance to reach the lower airway [17]. However, A. fumigatus can enter into sinus cavity and is the most common fungus isolated in sinonasal mucosa of non-invasive fungal rhinosinusitis patients [18,19]. For the pathognomic role of A. fumigatus, some pathologic conditions may need, such as many fungal conidia inoculation in sinuses, impaired mucociliary clearance, abnormal immunologic interaction between conidia and sinonasal mucosa or immune cells, and biofilm formation of inoculated conidia are required. Because the conidial number is important for biofilm formation, we used 1×105/mL of conidia, which is enough to form A. fumigatus biofilm [20]. According to the previous study, 1×104/mL or 1×106/mL conidia could make a biofilm with an easily disruptable structure [14]. After conidia seeding and germination, it takes about 48-72 h for biofilm formation and maturation [14,20]. So, we evaluate A. fumigatus biofilm formation for 72 h on primary human nasal epithelial cells.
Nasal epithelial cells are the first line defense organism against airborne allergen and microorganisms. Inhaled fungi interact with pattern recognition receptors of respiratory epithelial cells to activate innate and adaptive immune responses, which induce production of chemical mediators [13,21]. TLR2, TLR4, dectin-1, and long pentraxin 3 are representative receptors in the detection and clearance of fungal conidia [21]. A. fumigatus conidia or mycelial fragments induce cytokine production by activating intracellular signaling pathways [13,22]. IL-6 plays a role in acute and chronic inflammation and differentiation of B-cells. TGF-β1 acts as an anti-inflammatory agent and increases the fibrosis and tissue remodeling in sinonasal mucosa [23]. IL-8 is known as a neutrophil chemotactic factor. Neutrophils are responsible for killing and breaking down fungal hyphae [24]. In this study, A. fumigatus conidia enhanced IL-6, IL-8, and TGF-β1 expression which involved innate and adaptive immune responses in airway mucosa. At 48 h and 72 h, ZnO suppressed IL-8 production from primary human nasal epithelial cells. Although IL-8 is the only factor that eliminates fungi and protects host from fungal infection, decreased IL-8 may cause decreased neutrophil accumulation at sinonasal mucosa and then makes difficulties in removing A. fumigatus conidia, which may affect fungal differentiation and biofilm formation.
FB is most commonly found in the maxillary sinus, close to the molar and premolar teeth of the maxilla. Endodontic treatment of these teeth can make physical perforation and root filling materials can develop chemical inflammation of sinus mucosa [25]. Zinc, barium, and sulfur are the main constituents of endodontic filler. Zinc induces fungal growth and differentiation [10]. Endodontic sealers can enter into the maxillary sinus during endodontic treatment. The metallic components, such as ZnO, could play as a nidus for the development of FB with fungal growth and biofilm formation. A. fumigatus develop biofilm through the hyphae adhesion and embedding in an extracellular matrix [26]. When A. fumigatus was cultured with ZnO and primary human nasal epithelial cells, the biofilm and extracellular matrix formation increased significantly compared to the conidia cultured without primary human nasal epithelial cells. On the other hand, comparing A. fumigatus with ZnO and primary human nasal epithelial cells and without ZnO, development of the biofilm and extracellular matrix of A. fumigatus has so significant difference. There are no other studies that show the effects of ZnO in formation of A. fumigatus biofilm on primary human nasal epithelial cells, this might be the first study. ZnO is a commonly recognized risk factor for the development of sinus FB and could promote fungal growth and survival [7,10]. In contrast to our expectation, ZnO did not affect the formation of A. fumigatus biofilm. ZnO suppressed A. fumigatus-induced IL-8 production from primary human nasal epithelial cells, which inducing the accumulation of neutrophilic in sinonasal mucosa, an essential inflammatory cell that eliminates fungal elements [21,24]. Although, ZnO has a significant role in the development of FB, in-vitro study, ZnO alone could not influence the biofilm formation on primary human nasal epithelial cells. Our study design could not completely represent in-vivo conditions, such as air pollutants, mucociliary clearance system, the coexistence of bacteria or bacterial biofilms, the interaction of structural or inflammatory cells, and the role of chemical mediators in sinonasal mucosa. We cannot conclude that ZnO did not influence the development of sinus FB.
In conclusion, although this in-vitro study could not prove that ZnO promote or affect the development of A. fumigatus biofilm, our results suggest that ZnO exposed on primary human nasal epithelial cells may change microenvironmental defense system of sinonasal mucosa and induced abnormal immune responses against intranasally inoculated conidia of A. fumigatus.
ACKNOWLEDGMENTSSpecial thanks to Hun-Suck Seo for kindness in providing the fungus. This research was funded by the National Research Foundation of Korea (NRF) grant funded by the Korea government (Ministry of Science and ICT) (2019R1F1A1047757).
NotesAuthor Contribution Conceptualization: Seung-Heon Shin, Mi-Kyung Ye. Data curation: Seung-Heon Shin, Sang-Yen Geum, Hee-Jun Park. Formal analysis: Seung-Heon Shin, Mi-Kyung Ye, Jin-Woo Park, Hee-Jun Park. Funding acquisition: Seung-Heon Shin. Methodology: Seung- Heon Shin, Sang-Yen Geum. Supervision: Seung-Heon Shin. Writing— original draft: Sang-Yen Geum, Jin-Woo Park. Writing—review & editing: Seung-Heon Shin, Mi-Kyung Ye. Fig. 1.Cell viability effect of ZnO on primary human nasal epithelial cells at various concentration and times. Cell survival decreased significantly at 10 μg/mL ZnO. *p<0.05 compared with NC, n=5. NC, negative control; ZnO, zinc oxide. 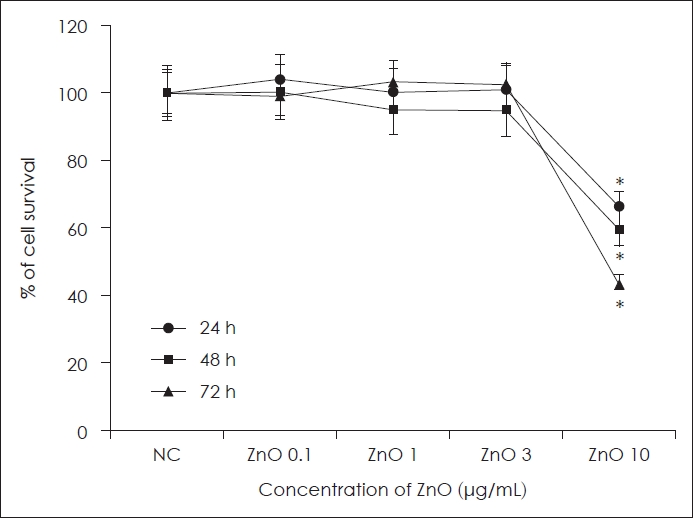
Fig. 2.Effect of ZnO and Asp on chemical mediator production in primary human nasal Epi. A. fumigatus enhanced IL-6, IL-8 and TGF-β1 protein production. After 48 h and 72 h incubation with 1 and 3 μg/mL ZnO, IL-8 protein production was significantly decreased. *p<0.05 compared with NC; †p<0.05 compared with Asp+Epi group, n = 7. NC, negative control; Asp, Aspergillus fumigatus; Epi, epithelial cells; ZnO, zinc oxide; IL, interleukin; TGF, transforming growth factor. 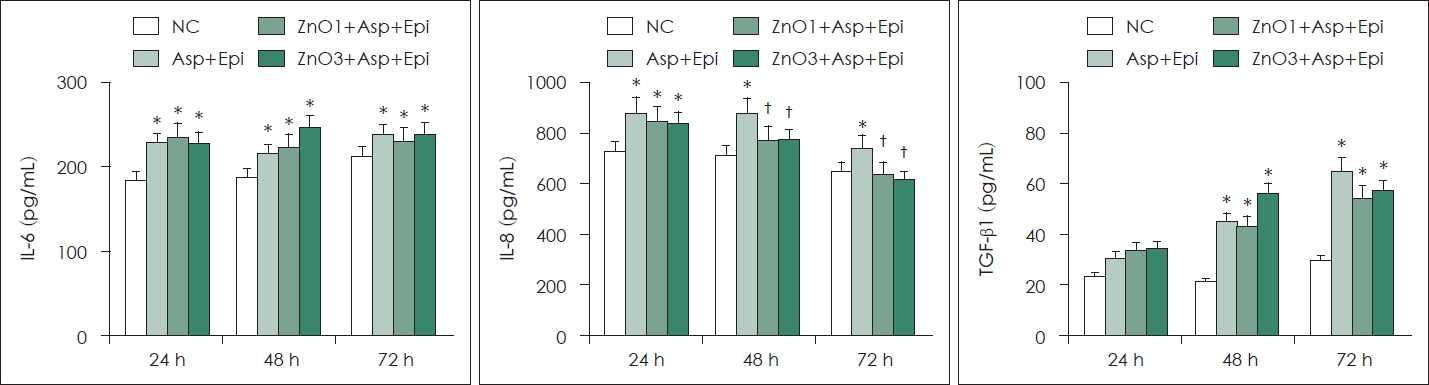
Fig. 3.Quantification of Asp biofilm in coculture of ZnO on the primary human nasal Epi. primary human nasal epithelial cells enhanced the development of fungal biofilm in compared with A. fumigatus culture without primary human nasal epithelial cells (Asp alone). However, coculturing of ZnO and A. fumigatus on primary human nasal epithelial cells did not affect biofilm dry weight (A), optical density for crystal violet (B), safranin (C), and concanavalin-A (D) staining. Epi+Asp: culture of Asp on the primary human nasal epithelial cells. ZnO1+Epi+Asp: culture of Asp with 1 μg/ml of ZnO on the primary human nasal epithelial cells, ZnO3+Epi+Asp: coculture of Asp with 3 μg/mL of ZnO on the primary human nasal epithelial cells, *p<0.05 compared with Asp alone, n=7. Asp, Aspergillus fumigatus; Epi, epithelial cells; ZnO, zinc oxide; OD, optical density. 
Fig. 4.Confocal scanning laser microscopy findings of Asp treated with ZnO. A-C show the representative confocal scanning laser microscopic findings after 48 hours. A. fumigatuson the epithelial cells (A, ×60 magnification), A. fumigatus with 1 μg/mL of ZnO (B, ×60 magnification), A. fumigatus with 3 μg/mL of ZnO (C, ×100 magnification). The arrowhead indicates Con-A stain binding to the fungal cell wall, the arrows indicates FUN-1 stain binding to metabolically active fungus, and yellow arrows indicate dual staining. Although ZnO did not affect fluorescence intensity of Con-A (D) and FUN-1 (E), Con-A and FUN-1 fluorescent intensity increased in a time-dependent manner. Epi+Asp: culture of Asp on the primary human nasal epithelial cells, ZnO1+Epi+Asp: culture of Asp with 1 μg/mL of ZnO on the primary human nasal epithelial cells, ZnO3+Epi+Asp: coculture of Asp with 3 μg/mL of ZnO on the primary human nasal epithelial cells. Epi, epithelial cells; Asp, Aspergillus fumigatus; ZnO, zinc oxide; Con-A, concanavalin A. 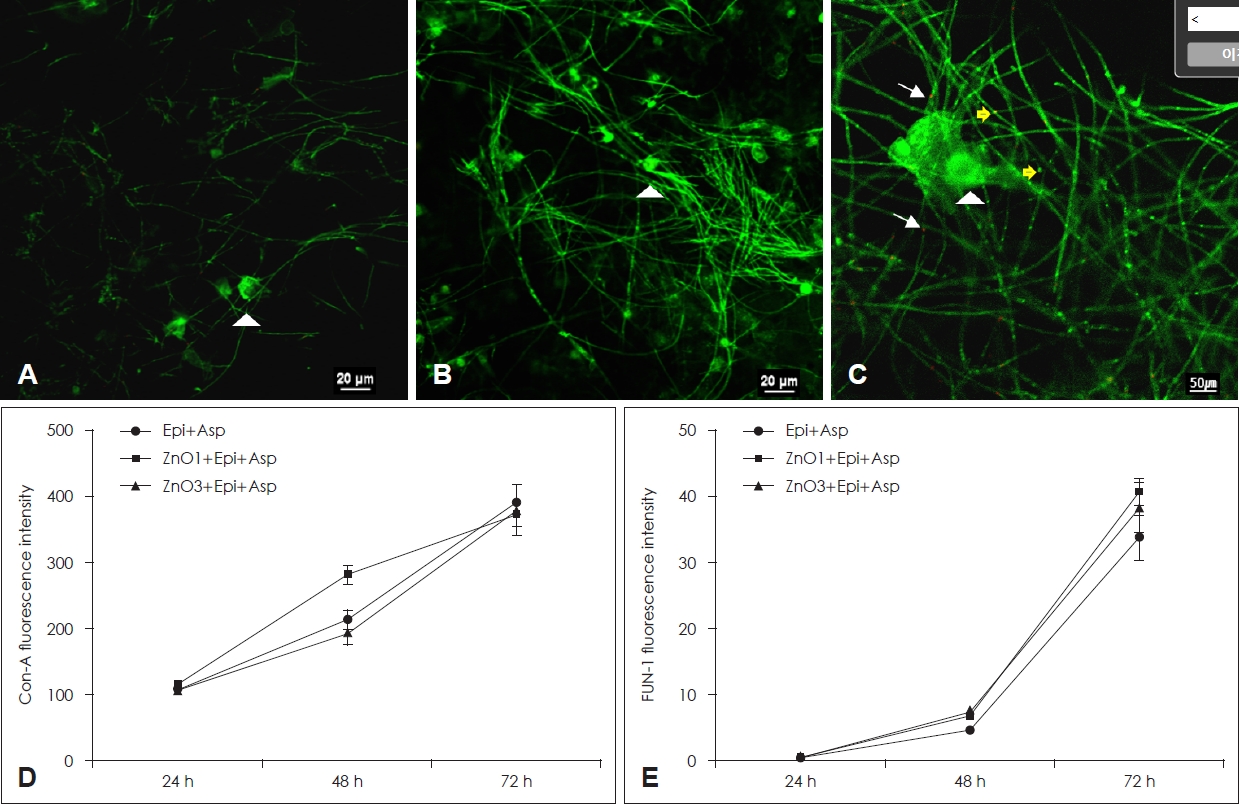
REFERENCES1. Lee JS, Shin SY, Lee KH, Kim SW, Cho JS. Change of prevalence and clinical aspects of fungal ball according to temporal difference. Eur Arch Otorhinolaryngol 2013;270(5):1673-7.
2. Kim DW, Kim YM, Min JY, Kim JW, Kim JK, Mo JH, et al. Clinicopathologic characteristics of paranasal sinus fungus ball: Ret rospect ive, mult icenter st udy i n Korea. Eu r A rch Otorhinolaryngol 2020;277(3):761-5.
3. Wani MK, Ruckenstein MJ, Parikh S. Magnetic resonance imaging of the paranasal sinuses: Incidental abnormalities and their relationship to patient symptoms. J Otolaryngol 2001;30(5):257-62.
4. Willinger B, Obradovic A, Selitsch B, Beck-Mannagetta J, Buzina W, Braun H, et al. Detection and identification of fungi from fungus balls of the maxillary sinus by molecular techniques. J Clin Microbiol 2003;41(2):581-5.
5. Mowat E, Williams C, Jones B, McChlery S, Ramage G. The characteristics of Aspergillus fumigatus mycetoma development: Is this a biofilm? Med Mycol 2009;47(1_suppl):S120-6.
6. Wang JH, Lee BJ, Jang YJ. Bacterial coinfection and antimicrobial resistance in patients with paranasal sinus fungus balls. Ann Otol Rhinol Laryngol 2010;119(6):406-11.
7. Wang X, Dong D, Cheng J, Fan X, Zhao Y. Relationship between biofilms and clinical features in patients with sinus fungal ball. Eur Arch Otorhinolaryngol 2015;272(9):2363-9.
8. Park GY, Kim HY, Min JY, Dhong HJ, Chung SK. Endodontic treatment: A significant risk factor for the development of maxillary fungal ball. Clin Exp Otorhinolaryngol 2010;3(3):136-40.
9. Scolozzi P, Perez A, Verdeja R, Courvoisier DS, Lombardi T. Association between maxillary sinus fungus ball and sinus bone grafting with deproteinized bovine bone substitutes: A case-control study. Oral Surg Oral Med Oral Pathol Oral Radiol 2016;121(6):e143-7.
10. Nicolai P, Mensi M, Marsili F, Piccioni M, Salgarello S, Gilberti E, et al. Maxillary fungus ball: Zinc-oxide endodontic materials as a risk factor. Acta Otorhinolaryngol Ital 2015;35(2):93-6.
11. Mensi M, Salgarello S, Pinsi G, Piccioni M. Mycetoma of the maxillary sinus: Endodontic and microbiological correlations. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2004;98(1):119-23.
12. Seidler MJ, Salvenmoser S, Müller FM. Aspergillus fumigatus forms biofilms with reduced antifungal drug susceptibility on bronchial epithelial cells. Antimicrob Agents Chemother 2008;52(11):4130-6.
13. Shin SH, Lee YH. Airborne fungi induce nasal polyp epithelial cell activation and toll-like receptor expression. Int Arch Allergy Immunol 2010;153(1):46-52.
14. Mowat E, Butcher J, Lang S, Williams C, Ramage G. Development of a simple model for studying the effects of antifungal agents on multicellular communities of Aspergillus fumigatus. J Med Microbiol 2007;56(Pt 9):1205-12.
15. Wasylnka JA, Simmer MI, Moore MM. Differences in sialic acid density in pathogenic and non-pathogenic Aspergillus species. Microbiology (Reading) 2001;147(Pt 4):869-77.
16. Ramage G, Williams C. The clinical importance of fungal biofilms. Adv Appl Microbiol 2013;84:27-83.
17. Kwon-Chung KJ, Sugui JA. Aspergillus fumigatus—What makes the species a ubiquitous human fungal pathogen? PLoS Pathog 2013;9(12):e1003743.
18. Shin SH, Ye MK, Lee YH. Fungus culture of the nasal secretion of chronic rhinosinusitis patients: Seasonal variations in Daegu, Korea. Am J Rhinol 2007;21(5):556-9.
19. Zhang J, Li Y, Lu X, Wang X, Zang H, Wang T, et al. Analysis of fungal ball rhinosinusitis by culturing fungal clumps under endoscopic surgery. Int J Clin Exp Med 2015;8(4):5925-30.
21. Croft CA, Culibrk L, Moore MM, Tebbutt SJ. Interactions of Aspergillus fumigatus conidia with airway epithelial cells: A critical review. Front Microbiol 2016;7:472.
22. Sharon H, Amar D, Mircus G, Shadkchan Y, Shamir R, et al. PrtT-regulated proteins secreted by Aspergillus fumigatus activate MAPK signaling in exposed A549 lung cells leading to necrotic cell death. PLoS One 2011;6(3):e1003743.
23. Little SC, Early SB, Woodard CR, Shonka DC Jr, Han JK, Borish L, et al. Dual action of TGF-beta1 on nasal-polyp derived fibroblasts. Laryngoscope 2008;118(2):320-4.
24. Hohl TM, Feldmesser M. Aspergillus fumigatus: Principles of pathogenesis and host defense. Eukaryot Cell 2007;6(11):1953-63.
|
|
||||||||||||||||||||||||||||||||||||||||

 |
 |