쉽게 오진될 수 있는 생명을 위협하는 비부비동 침습성 아스페르길루스증
Life-Threatening Sinonasal Invasive Aspergillosis Easily Misdiagnosed
Article information
Trans Abstract
Background and Objectives
Invasive fungal sinusitis is extremely rare. Yet the consequences of this infection may be fatal if not diagnosed correctly at an early stage. We discuss three cases of Aspergillosis sphenoid sinusitis that were misdiagnosed and have led to fatal consequences.
Subjects and Method
This is a retrospective analysis of cases diagnosed as invasive fungal sinusitis of sphenoid sinus.
Results
All three cases were of men who had immunocompromised medical histories that included leukemia and diabetes mellitus. The patients presented symptoms that included fever, chronic headache, weakness on one side, and facial edema. In the first case, CT showed no suspicious lesions that indicated fungal infection. However, five days after being prescribed antibiotics, the patient suffered obstruction of the right internal carotid artery (ICA) and right cavernous sinus. In the second case, CT showed total obstruction in the left ICA and a soft tissue mass in the left sphenoid sinus that extended and involved the left cavernous sinus. In the last case, CT showed soft density progression in the right sphenoid sinus with bone erosion when compared to images taken eight days earlier. In each of these cases, we performed emergency endoscopic sinus surgery. One case was confirmed to be invasive aspergillosis by histology whereas the other two cases were suspected to be invasive aspergillosis based on their past medical histories, clinical findings and image studies.
Conclusion
Clinicians must take great care to rule out invasive aspergillosis in immunocompromised patients with sinusitis.
Introduction
Fungal pathogens are well known to cause severe and fatal opportunistic infections in immunocompromised patients [1]. Among fungal pathogens, invasive aspergillosis in particular, is a leading cause of death due to its rapid infection progress and intracranial spread [2]. The most frequent species isolated in human infections, and thus of medical importance, is Aspergillus fumigatus[3]. Invasive Aspergillus infection may occur through various sites such as the respiratory tract, broken skin barrier, cornea and the ear. In this paper, the infection site of interest is the respiratory tract, especially the sinuses [4]. The ability to sporulate abundantly into the air in the form of spores and conidia enables Aspergillus infection of the sinuses by passing airflow [1,2]. However, although the cases in this paper were diagnosed as Aspergillus sphenoid sinusitis, it should be noted that sinus infection by fungus is relatively rare, due to the lack of airflow and the anatomic location of the sinuses [2].
The clinical manifestations of Aspergillus sphenoid sinusitis differ from other fungal infections of the sinuses. While most fungal sinusitis presents with symptoms similar to that of bacterial infections, such as nasal discharge, sinus pain, fever, headache, etc., fungal infection in the sphenoid sinuses may present with additional visual symptoms such as “retroorbital pain, diplopia, exophthalmos, and blindness [2].” In addition, due to the anatomical proximity of the sphenoid sinuses to the visual nerves and carotid artery, complications range from visual disturbances to brain abscess, stroke and even death due to the involvement of the carotid artery [4]. Through the three cases presented in this study, we emphasize the subtle, but important differences that may be fatal in the management of fungal infection in the sphenoid sinuses.
Subjects and Methods
This is a retrospective analysis of invasive fungal sinusitis of sphenoid sinus cases in the department of otolaryngology of our institution. Three cases of aspergillosis sphenoid sinusitis with severe or fatal consequences reported in our hospital from 2017 to 2018 were included. In all cases patients were immunocompromised - either had received chemotherapy or had uncontrolled diabetes. All patients underwent emergency endoscopic sinus surgery (ESS) with one surgeon. This study was approved by the Institutional Review Board of our National University Hospital Committee, the approval number of the ethics committee: CUH 2020-08-015.
Results
Case I
A 58-year-old male was admitted to our hospital for consolidation therapy. He had been in remission from acute myeloid leukemia for 3 months. The patient had suddenly developed persistent fever, headache and facial edema during hospitalization. The hematologist-oncologist suspected fungal infection and consulted with the Department of Otorhinolaryngology. The patient was under diabetes medication, and had received ESS 4 years earlier due to inverted papilloma in the left sinus. There were no specific findings during physical examination, including nasal endoscopy. Paranasal sinus (PNS) CT findings also showed no suspicious lesions that might indicate fungal infection (Fig. 1A-C). Therefore, antibiotics and nasal douching were prescribed for the follow-up period. Five days later, the patient showed chemosis and limitation of the entire visual field in the right eye. Visual acuity measured 0.6 in the right eye and 1.2 in the left eye. On the follow-up PNS CT scan 12 days after the initial scan, sinusitis showed an overall improvement, but the lesion in the right sphenoid sinus remained and the right cavernous sinus showed a density change that indicated obvious obstruction of the right internal carotid artery (ICA) and right cavernous sinus (Fig. 1D and E). Preoperative diagnosis was ‘chronic sinusitis (both),’ ‘orbital apex syndrome (right),’ and ‘suspicion of fungal infection (right).’ Emergency ESS was performed and amphotericin B was initiated with suspicion of mucormycosis. Postoperative brain inner ear MRI with contrast enhancement was performed. This revealed total occlusion in the right cavernous ICA (Fig. 1F). On the 6th day after surgery, invasive aspergillosis was confirmed on biopsy (Fig. 1G and H) and the antifungal agent was changed to voriconazole. Twentyone days after surgery, the patient’s extraocular muscle movement (EOM) was restored to normal, and on the 45th day, endoscopic examination showed no sign of recurrence. A follow-up MRI scan taken 1 month later showed that total occlusion in the right cavernous ICA still existed but mild improvement in thrombophlebitis of the right cavernous sinus was noted. The patient was transferred to another hospital after treatment with the antifungal agent for 3 months and lived for a further 15 months without recurrence.
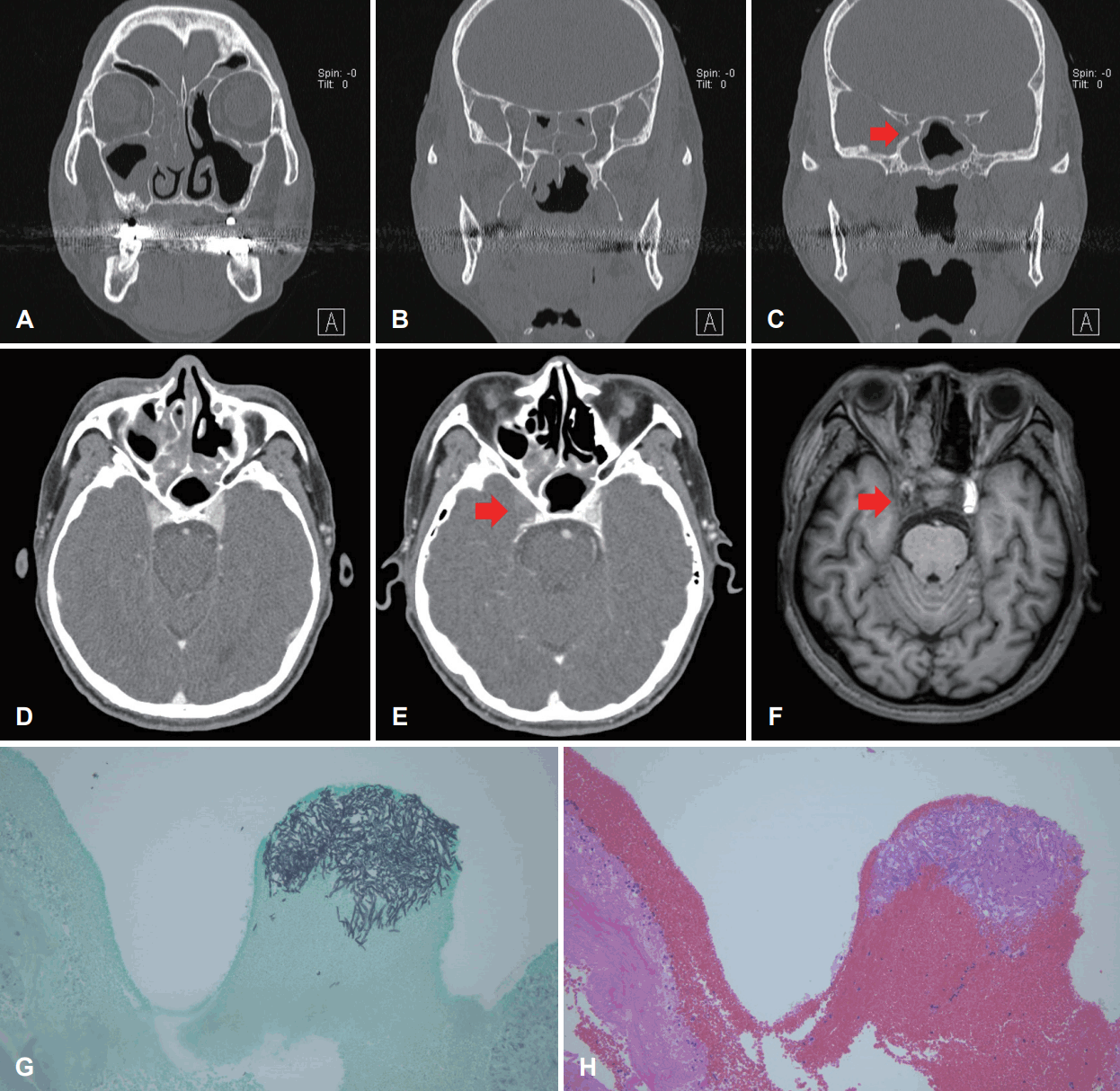
Consecutive paranasal CT, MRI and pathologic findings of a 58-year-old male who had a history of acute myeoloid leukemia in Case I. A: CT scan showing right ethmoidal sinusitis with soft tissue density in right ethmoid sinus. B: CT scan showing sphenoidal sinusitis with soft tissue density in both sphenoid sinuses. C: CT scan showing soft tissue density in right sphenoid sinus with erosion in skull base (red arrow). D and E: Follow-up CT, 12 days after initial CT. Sinusitis showed overall improvement; however, there was obvious obstruction of the right ICA and right cavernous sinus (red arrow). F: Brain paranasal MRI with contrast enhancement suggestive of thrombophlebitis in the right cavernous ICA (red arrow). G: Gomori methenamine-silver stain ×200. Presence of intravascular fungal hyphae, morphologically consistent with invasive aspergillosis. H: Hematoxylin and eosin stain ×200. Presence of intravascular fungal hyphae, morphologically consistent with invasive aspergillosis. ICA, internal carotid artery.
Case II
A 63-year-old male visited the emergency room (ER) complaining of a change in mental wellbeing and right-sided weakness. He had a medical history of hypertension and diabetes mellitus, and was currently taking medication for chronic headaches prescribed by our Department of Neurology. The patient had taken a brain MRI scan 2 months earlier due to chronic headache, and the radiologists interpreted it as a simple chronic sphenoid sinusitis (Fig. 2A and B). A week earlier before his visit to our ER, the patient had been admitted to our Department of Endocrinology due to uncontrolled diabetes and during his hospitalization, he received otolaryngology treatment due to headache suspicious of originating from sinusitis. At the time, the otolaryngologist recommended PNS CT, but the patient had refused to proceed. Upon neurological examination during his present visit in the ER, his mental status was assessed as deep drowsy, with right upper extremity motor: 3/5 grade, right lower extremity motor: 3/5 grade, left upper extremity motor: 4/5 grade, and left lower extremity motor: 4/5 grade. Both feet showed positive Babinski sign with accompanying neck stiffness. A contrast enhanced brain MRI scan was taken, and was reported to show an acute infarct in the left thalamus, left temporal lobe medial portion, internal capsule, left basal ganglia, and left occipital lobe, as well as a density change in the left sphenoid sinus and obstruction in the left ICA (Fig. 2C-E). Spiral CT 3-D common carotid angio + brain perfusion results showed total obstruction in the left ICA, and the left middle cerebral artery was supplied by the anterior communicating artery and posterior communicating artery. During his initial stay in the ER, the neurologist suspected invasive fungal sinusitis and referred him to our department for further evaluation and management.

Consecutive MRI, paranasal CT and pathologic findings of a 63-year-old male who had a history of hypertension and diabetes in Case II. A: Brain MRI, left sphenoid sinus showing hypointensity compared to surrounding bone density on T1-weighted images. B: Brain MRI, marked hypointensity of the left sphenoid sinus on T2-weighted images. C-E: Brain MRI showing acute infarct in left thalamus, left temporal lobe medial portion, internal capsule, left basal ganglia, left occipital lobe and left ICA obstruction. Left ICA obstruction observable with destruction in the medial part of the bone. F and G: CT scan showing left cavernous sinus involvement due to left sphenoid sinus fungal sinusitis with obstruction seen in the left ICA. H: Aspergillosis invades adjacent mucosa (arrows). I: High-power image of hyphae of aspergillosis. Original magnification: H, H&E stain ×200; I, H&E stain ×400. ICA, internal carotid artery; H&E, hematoxylin and eosin.
During endoscopic examination, a whitish discharge was found in both nasal cavities. A PNS CT was taken, and a soft tissue mass was found in the left sphenoid sinus extending to and involving the left cavernous sinus (Fig. 2F and G). The radiologist diagnosed left ICA obstruction with left cavernous sinus involvement, due to mucocele or sinus fungal sinusitis in the left sphenoid sinus. Invasive fungal sinusitis was suspected and an emergency ESS was performed. During the surgery, a fungal ball was found in the left sphenoid, with dehiscence to the ICA wall. In order to confirm invasive fungal sinusitis, a biopsy was taken from the sphenoid wall. Voriconazole was given as antifungal agent, and the biopsy results reported aspergillosis (Fig. 2H and I). The patient has been in a coma for over 5 months after the surgery, and still remains in the neurological intensive care unit even at the time of writing of this paper.
Case III
A 61-year-old male visited our outpatient clinic due to rightsided headache with throbbing and tightening pain. He had visited the ER twice over the previous 2 weeks with those symptoms and a brain MRI had been performed which yielded no specific findings. He had merely received medication from the neurology department and had been discharged. The patient had no diplopia or EOM limitation upon physical examination, and had no nasal symptoms. He had a medical history of diabetes and had received chemotherapy for 5 months due to leukemia diagnosed a year earlier. Nasal endoscopic examination showed a white silky discharge in the right sphenoid sinus, and the primary physician suspected right sphenoid sinusitis with fungal ball, prescribing PNS CT with a follow-up appointment.
Four days after visiting our outpatient clinic, the patient visited the neurology clinic due to persistent headache and was admitted to control his symptoms. At admission, the patient was alert and showed no abnormal neurologic signs. During hospital day 2, the patient showed fever and laboratory test results reported white blood cell 1.61×103/μL, neutrophil 590/μL, high-sensitivity C-reactive protein 117.8 mg/L, and erythrocyte sedimentation rate 43 mm/h. Upon reviewing his past medical records, he was found to have shown continuous neutropenia after receiving chemotherapy.
The neurology department consulted the hemato-oncology department to rule out neutropenic fever, and the patient was transferred to the hemato-oncology department for treatment. Laboratory test results for Aspergillus antigen were positive, and the patient was referred to our department to rule out invasive fungal sinusitis. A PNS CT was performed immediately, and soft density progression was seen in the right sphenoid sinus with bone erosion in the superolateral wall when compared to images taken 8 days earlier at our outpatient clinic (Fig. 3A and B). In addition, mucosal thickening had developed in the left sphenoid sinus which had not been present in previous images.
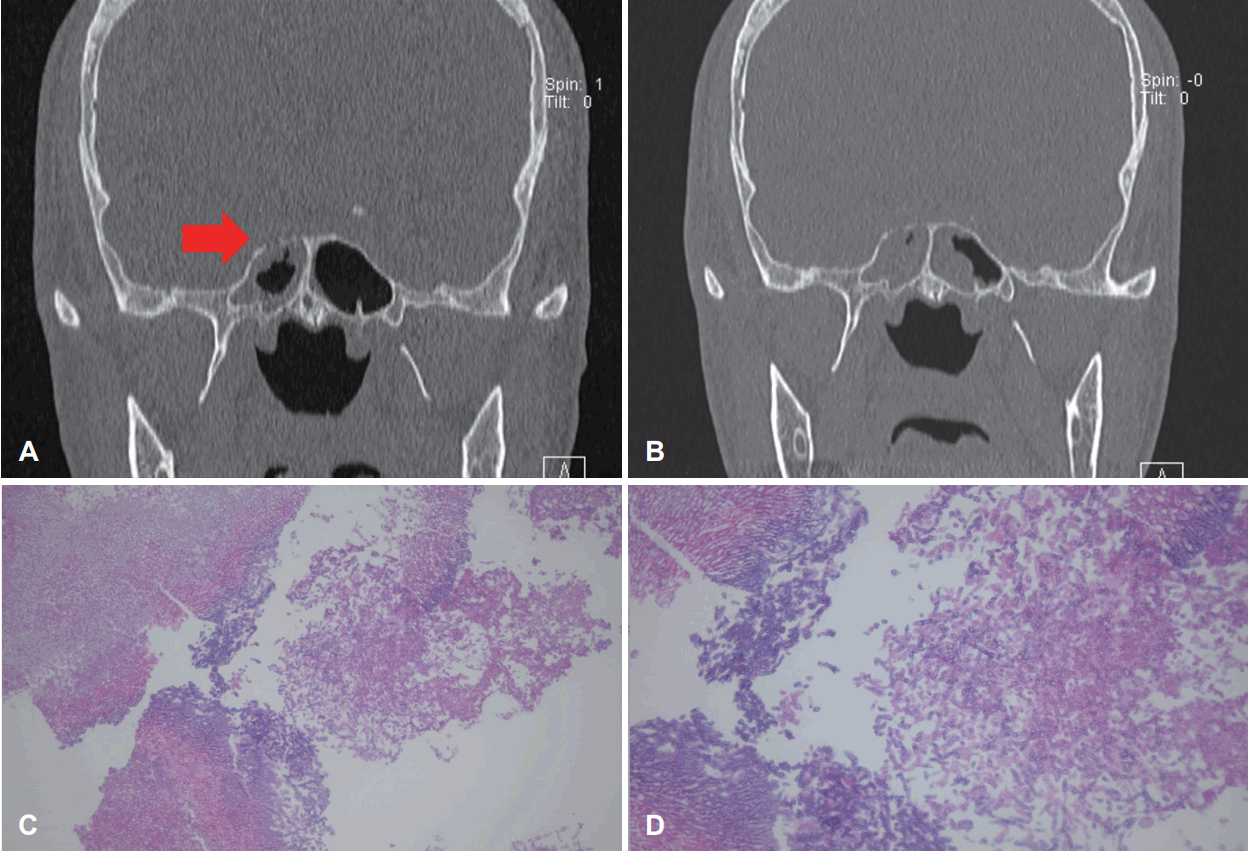
Consecutive paranasal CT and pathologic findings of a 61-year-old male who had a history of leukemia and diabetes in Case III. A: CT scan showing Mucosal thickening in the right sphenoid sinus with erosion in the skull base (red arrow). B: 8 days later. Progression of the involved site in the right sinus with mucosal thickening in the left sphenoid sinus. C and D: Presence of fungal hyphae, morphologically consistent with aspergilloma. Original magnification: C, H&E stain ×200; D, H&E stain ×400. H&E, hematoxylin and eosin.
Emergency ESS was performed and the whole sinus revealed pale cadaveric mucosa. After surgery, Voriconazole was administered as antifungal agent. Histological examination revealed fungal hyphae, morphologically consistent with aspergilloma (Fig. 3C and D). Three days after surgery, diplopia with limitation in the right lateral gaze of the right eye had developed. The patient received antifungal agent for 38 days after surgery and was discharged. He is still alive at the time of writing (28 months after surgery).
Discussion
Invasive fungal sinusitis may be classified into acute and chronic forms, and ‘acute invasive’ fungal sinusitis carries a high mortality rate. Acute invasive fungal sinusitis occurs as opportunistic infections in leukemia, lymphoma, aplastic anemia, acquired immune deficiency syndrome, organ transplant and other immunocompromised patients [5]. The most common fungal type is Aspergillus spp., which is angio-invasive, and spreads hematogenously. Clinical manifestations include fever, facial pain, nasal congestion, epistaxis, proptosis, visual disturbance, headache, mental status changes, and seizures. Also, death resulting from rapid orbital and intracranial spread is characteristic of fungal infection.
What should be noted in our first case is that fever, headache, and abnormalities seen in imaging studies should trigger caution, especially when present in immunocompromised patients. Our patient had sudden fever, headache, and facial edema and was first referred to the hematologist-oncologist and then to our department for differential diagnosis. There was no abnormality upon physical examination and endoscopic examination, and no suspicious lesion on PNS CT scans that might suggest fungal infection. Only after a careful, close observation of the PNS CT, can thickening of the sphenoid wall and slight bone erosion of the skull base be noted (Fig. 1C). Though physical and endoscopic examination, as well as imaging studies may not indicate a fatal outcome, we suggest attentive treatment if immunocompromised patients show signs of infection such as persistent fever.
In the second case, the patient presented with non-specific symptoms such as chronic headache which we were unable to associate with fungal sinusitis. In addition, physical examination and nasal endoscopy showed no findings that might have indicated fungal sinusitis. Upon examining his medical records more closely, the patient was noted to have had persistent headaches for the past year, and had received evaluation at our neurology department when the symptoms began to worsen 3 months earlier. Upon brain MRI, there were no other suspicious findings other than a fungal ball in the sphenoid sinus. The radiologist at the time diagnosed chronic sinusitis in the sphenoid sinus. Since the neurologist could not find any lesions in the brain, the patient was given medication merely for pain control.
As his symptoms persisted, the patient visited other local outpatient clinics and, upon blood-glucose monitoring, was found to have hyperglycemia. He was referred to our Department of Endocrinology for blood-glucose control, and was admitted for a week. During that week, the patient was referred to our department for headache evaluation, and was found to have no symptoms other than rhinorrhea, post-nasal drip and hyposmia due to chronic right nasal obstruction. No specific findings were present upon endoscopic exam, and since the patient refused to take another PNS CT, he was discharged for follow-up at our outpatient clinic. It was only 5 days after his discharge that he arrived back at our ER again due to a change in his mental status.
In retrospect, careful and meticulous observation of the brain MRI may have improved our diagnosis since the left sphenoid sinus showed a density change. Fungal sinusitis may have been suspected from these findings (Fig. 2A and B).
What we could improve to make a more accurate diagnosis in the future, is to pay close attention to the patient’s underlying medical history and previous images. Since the patient in our case had received treatment for uncontrolled diabetes, we should have kept in mind that he was immunocompromised, and thus could have clinical problems related to such a condition. Therefore, for future reference, we emphasize the importance of fully taking into account the patient’s underlying medical condition, however subtle or irrelevant it may seem to the current clinical condition of the patient. Doing so would not only improve the accuracy of the diagnosis, but also help in interpreting test results that are vague and show discordance with the clinical symptoms.
The patient in our last case was also immunocompromised and had uncontrollable localized headache. The primary physician suspected fungal infection and evaluated with a diagnosis of fungal infection in mind. However, the condition of the patient deteriorated rapidly while evaluation was in progress. Although emergency surgery was performed immediately after symptoms seemed to worsen, the infection had already progressed to a state that could not be corrected completely by surgery. Reviewing the case, when the patient had experienced uncontrollable headache even after seeing the neurologist, fungal infection could have been suspected because the patient had been immunocompromised for years. Also, if immediate CT evaluation had been performed when the patient had visited our outpatient clinic, treatment would have been administered even earlier, and better treatment outcomes would have been possible. Gillespie and O’Malley [6] reported that invasive fungal sinusitis is prevalent in people with low immunity and a diagnosis of invasive fungal sinusitis may be delayed due to ambiguous early symptoms. Therefore, it is most important to examine patients with immunocompromised status very closely and with great suspicion.
From the three cases reported here, we emphasize the fatal path that may follow sphenoid fungal infection in immunocompromised patients. We also recommend that other clinical fields that encounter immunocompromised patients should review these cases carefully so that, in future, such clinical features may prompt suspicion of invasive fungal sinusitis.
This study had the following limitations. 1) It was a retrospective study; however, invasive fungal sinusitis is an ethical problem due to its fatal nature and it cannot be studied prospectively. 2) This study is based on the experience of a single center, single surgeon.
Even if there is no suspicion of invasive aspergillosis upon histologic examination, it is of utmost importance to identify any possible history of immunocompromise. If the patient is immunocompromised, CT imaging should be performed to identify any signs of sinusitis, even though initial nasal endoscopy may seem normal. If sinusitis is found with imaging studies, aggressive surgical management is important. If the patient is immunocompromised and aspergillosis is found upon biopsy, clinicians should take a cautious approach and suspect invasive aspergillosis regardless of whether there is no sign of vessel invasion upon biopsy.
Acknowledgements
This work was supported by the NRF grant funded by the Korean Government (MSIT) (No. 2021R1G1A1094681) and by project for Industry-Academic Cooperation Based Platform R&D funded by the Korea Ministry of Small and Medium Enterprises (SMEs) and Startups in 2021 (No. S3017921). This research was supported by a Young Medical Scientist Research Grant through the Seokchunnanum Foundation (SCY2113P) and by the fund of the Biomedical Research Institute, Jeonbuk National University Hospital. This work was supported by the Ministry of Health & Welfare, Republic of Korea (grant number: HI22C1124).
Notes
Author Contribution
Conceptualization: Jong Seung Kim. Data curation: Yeon Seok You. Formal analysis: Yeon Seok You. Investigation: Ji Hoon Koh. Methodology: Ji Hoon Koh. Project administration: Yeon Seok You. Resources: Byeong Jin Kim. Software: Byeong Jin Kim. Supervision: Jong Seung Kim. Validation: Eun Jung Lee, Sam Hyun Kwon. Visualization: Eun Jung Lee, Sam Hyun Kwon. Writing—original draft: Jong Seung Kim. Writing—review & editing: Jong Seung Kim.
