수면호흡장애 환자에서 체질량지수가 약물유도수면내시경상 상기도폐쇄에 미치는 영향
Effect of Body Mass Index on Upper Airway Obstruction as Revealed by Drug-Induced Sleep Endoscopy in Patients With Sleep-Disordered Breathing
Article information
Trans Abstract
Background and Objectives
Drug-induced sleep endoscopy (DISE) usefully determines the sites of airway obstruction in patients with sleep-disordered breathing. It is widely accepted that obesity increases obstructive sleep apnea. However, no study has explored how and where obesity causes obstructions. In this study, we described the patterns of upper airway obstruction revealed by DISE in patients of various body mass indices (BMIs) with sleep-disordered breathing.
Subjects and Method
All subjects had sleep-disordered breathing and underwent DISE. Endoscopic findings at the retropalatal (upper lateral, upper anteroposterior [AP]) and retrolingual (lower lateral, lower AP) levels were graded; obstruction was complete (2), partial (1), or none (0). Subjects with BMI <25 (<25 group), 25≤BMI<30 (25<30 group), and ≥30 kg/m<sup>2</sup> (>30 group) were compared using a dummy variable; to this end, we employed R ver. 4.0.5.
Results
For the total of 153 patients reviewed, the mean age was 43.1±12.2 years and the mean BMI was 26.0±3.4 kg/m<sup>2</sup>. At the retropalatal AP level, the DISE grade was significantly higher in the 25<30 group than in the <25 group (p;=0.029) but not in the >30 group (p;=0.248). At the retropalatal lateral level, significant increases were evident in both of the higher BMI groups (p;=0.06 and p;=0.024, respectively). No significant relationship was found at the retrolingual level.
Conclusion
In terms of the retropalatal AP and lateral diameters, a higher BMI is associated with a greater incidence of more severe obstruction.
Introduction
Obstructive sleep apnea syndrome (OSAS) is a common sleep disorder characterized by frequent interruption of breathing because of upper airway obstruction occurring during sleep. This causes night-time hypoxemia, frequent awakenings, excessive daytime sleepiness, and increases the risk for cardiovascular and cerebrovascular complications [1-3].
The severity of OSAS is affected by age, sex, body mass index (BMI), and sleep posture. Especially, it is widely accepted that OSAS risk correlates well with BMI [4,5]. Peppard, et al. [6] found that a 10% increase in weight predicted a 6-fold increase in the risk of developing moderate to severe sleep-disordered breathing. Tufik, et al. [7] reported moderate to severe OSAS (apnea-hypopnea index [AHI] ≥15) in 11% of normal-weight men, 21% of those who were overweight (BMI 25-30 kg/m2), and 63% of those who were obese (BMI >30 kg/m2).
Several studies that have used CT and MRI have reported structural changes in the upper airway caused by obesity, but such static tests cannot reflect real-time airway status during sleep [8-10]. It has been difficult to ascertain exactly when, where, and how obstructions develop during sleep in obese OSAS patients.
In 1991, Croft and Pringle developed “sleep nasoendoscopy” for upper airway evaluation during sedation. Subsequently, Kezirian coined the term “drug-induced sleep endoscopy (DISE)” and this is now widely performed. The limitations of DISE are that sleep is artificially induced and the observation time is limited, but it mimics natural sleep more so than do other diagnostic tools.
Most studies that have explored upper airway obstructions by BMI status have enrolled only Caucasians. Asians differ in terms of lifestyle, diet, and body type; BMI-induced obstructions in Asians thus require special study. Here, we explored the extents and patterns of upper airway obstruction during DISE sleep in Korean OSAS patients with various BMIs.
Subjects and Method
Patient selection
We retrospectively evaluated 271 patients (mean age 42.84 years; range 17 to 73 years) who underwent full-night polysomnography and were diagnosed with OSAS (AHI >5) at Busan Saint Mary’s Hospital ear, nose, and throat department from 2013 to 2020. Prior to DISE, a medical history was documented for all patients and they also received examinations of the ear, nose, and throat. As absolute contraindications for DISE, those with American Society of Anesthesiologists class 4, pregnancy and with allergy to drugs used for DISE were excluded and morbidly obese patients, a relative contraindication, were also not eligible. To prevent bias induced by anatomical abnormalities of the upper airway, patients with retrognathia, micrognathia, and macroglossia were excluded. In addition, tonsil and epiglottis are factors that contribute a lot to airway obstruction even though they are not related to obesity [11,12], so only patients with a grade of 0 tonsil and epiglottis on the DISE were included. Ultimately, 153 patients were enrolled and divided into three groups according to BMI (BMI <25 [normal group], 25≤BMI<30 [overweight group], and BMI ≥30 kg/m2 [obese group]). The study was approved by the Institutional Review Board of Busan Saint Mary’s Medical Center (Approval No. BSM-2021-12).
DISE
With the patient supine, DISE was performed under respiratory monitoring by an anesthesiologist in the operating room. Sleep was induced via intravenous administration of low-dose midazolam (0.07 mg/kg), acting only as a sleep inducer and not as a sedative. After the patient fell asleep, a flexible videolaryngoscope 4 mm diameter was inserted into the nose. Video images were later evaluated by a single otolaryngologist.
Classification of DISE findings
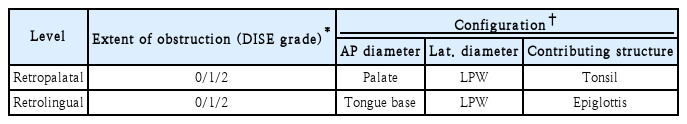
We divided the upper airway into retropalatal (the region posterior to the soft palate) and retrolingual (the region of the pharynx posterior to the vertical portion of the tongue) areas. The retropalatal area was subdivided into the palate (the anteroposterior [AP] diameter) and the lateral pharyngeal wall (the lateral diameter). The retrolingual area was subdivided into the tongue base (AP diameter) and the lateral pharyngeal wall (lateral diameter). As structures contributing to airway obstruction, tonsil and epiglottis were also evaluated separately. Airway obstruction (DISE grade) was classified as none (grade 0, <50%, only a decrease in diameter with respiration, but no obstruction at all), partial (grade 1, 50%-75%), and complete (grade 2, >75%) (Table 1) [11].
Statistical analysis
All statistical analyses were performed using R ver. 4.0.5 (R Foundation for Statistical Computing, Vienna, Austria). To compare categorical DISE grades between the BMI groups, we employed a dummy variable. Dummy variable regression analyses were used to explore the relationships between BMI and DISE grade. A p-value<0.05 was considered significant; mean values are presented.
Results
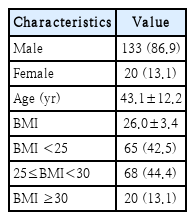
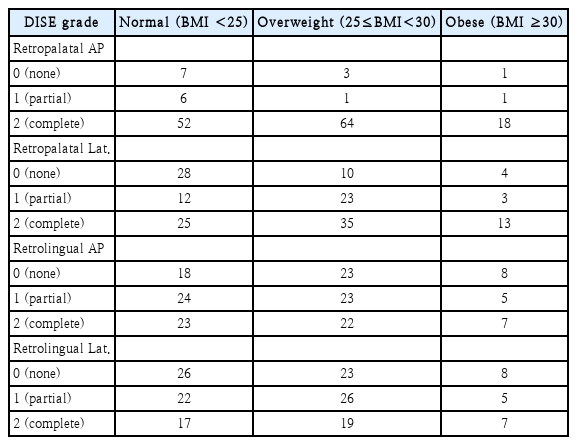
We reviewed data on 153 patients of mean age 43.1±12.2 years and mean BMI 26.0±3.4 kg/m2 (Table 2). There were 65 (42.5%), 68 (44.4%), and 20 (13.1%) patients in the normal, overweight and obese group, respectively. Table 3 lists the DISE grades by location and BMI group. Compared with other levels, severe obstructions were more frequent at the retropalatal AP level. At the retropalatal lateral level, the frequency of severe obstruction increased as the BMI increased.
Figs. 1 and 2 show the relationships between BMI and DISE grade. The overweight group and obese group were compared to the normal group and each increase in the mean value of a DISE grade is indicated by a ‘b’ value, the slope of a line. At the retropalatal AP level, the DISE grade increased in the overweight group (p=0.029) but not in the obese group (p=0.248) (Fig. 1). At the retropalatal lateral level, significant increases were evident in both groups (p=0.006 and 0.024, respectively) (Fig. 1). At the retrolingual level, no significant relationship was found (Fig. 2).
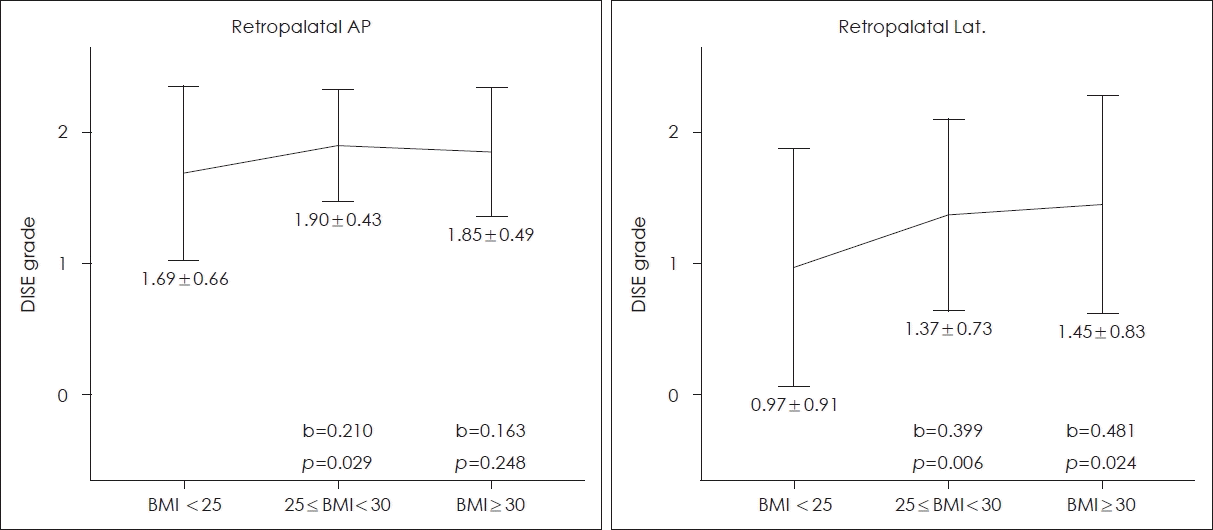
Severity of obstruction at the retropalatal level by the BMI. The mean and standard deviation of DISE grade for each group are shown below each vertical line. The overweight (25≤BMI<30) group and obese (BMI <30) group were compared to the normal (BMI <25) group and each increase in the mean value of a DISE grade is indicated by a ‘b’ value, the slope of a line. At the retropalatal AP level, the DISE grade increased in the overweight group (p=0.029) but not in the obese group (p=0.248). At the retropalatal lateral level, significant increases were evident in both groups (p=0.006 and 0.024, respectively). AP, anteroposterior; Lat., lateral; BMI, body mass index (kg/m2); DISE, drug-induced sleep endoscopy.
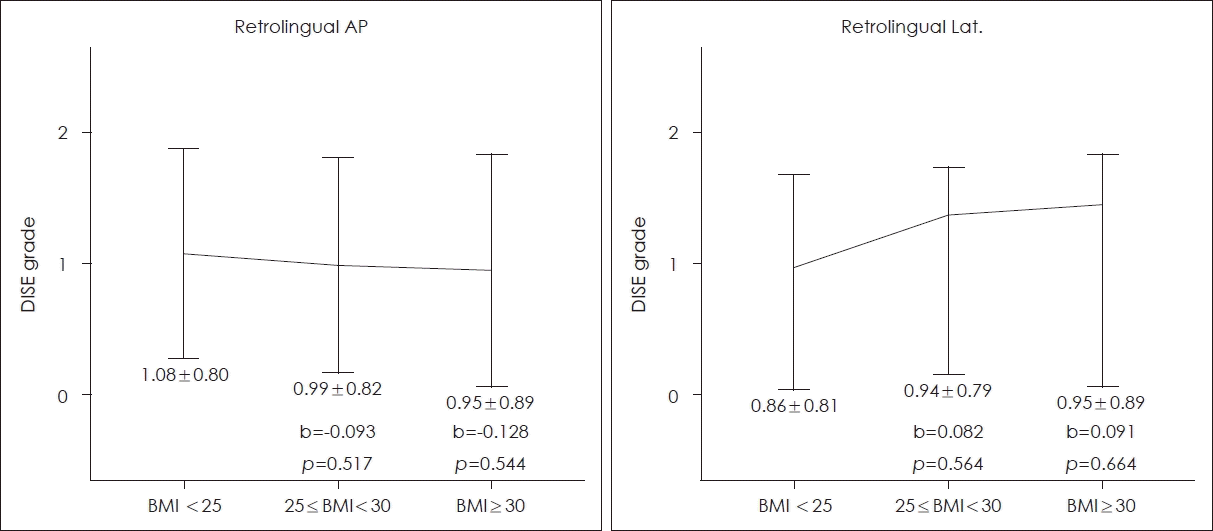
Severity of obstruction at the retrolingual level by the BMI. The mean and standard deviation of DISE grade for each group are shown below each vertical line. The overweight (25≤BMI<30) group and obese (BMI <30) group were compared to the normal (BMI <25) group and each increase in the mean value of a DISE grade is indicated by a ‘b’ value, the slope of a line. At the retrolingual level, no significant relationship was found. AP, anteroposterior; Lat., lateral; BMI, body mass index (kg/m2); DISE, drug-induced sleep endoscopy.
Discussion
Compared to other modalities, DISE more practically assesses obstructive sleep apnea. Although CT and MRI can reveal structural changes in the upper airway caused by obesity, these methods are static and reflect airway status at only one moment in time (and not during sleep) [8-10]. Although drug-induced sleep is not identical to actual sleep, DISE approaches a more natural physiological sleep state than any other diagnostic tool currently available. It determines the location of an obstruction and aids surgical decision-making.
When using sedatives, the depth of anesthesia is very important. Excessive sedation may decrease upper airway muscle tone and increase OSAS [13]. To date, there has been no standard protocol, but the ideal concentration of sedatives varies from individual to individual. We performed patient-specific procedures in collaboration with anesthesiologists. Sleep was induced using low-dose midazolam, which acts only as a sleep inducer and not as a sedative. Minimal doses of drugs were injected using targeted-controlled infusion as the drug delivery system under the supervision of an anesthesiologist.
Careful DISE is important, but the manner in which the test data are presented is also important. The VOTE classification focuses on specific structures involved in obstruction. However, this tends to oversimplify the upper airway structure, rendering surgical decision-making difficult. We thus used the classification of Koo, et al. [11] (Table 1). Areas of possible obstruction were divided into AP and lateral, higher (retropalatal) and lower (retrolingual) levels, facilitating a more intuitive description of upper airway obstructions from the surgical viewpoint.
In terms of the retropalatal AP and lateral diameters, a higher BMI was associated with more severe obstruction, except in the obese group at the retropalatal AP. In other words, upper airway obstruction during sleep at the retropalatal level tends to increase as the BMI increases. Similar findings were reported by Wong, et al. [14] and Lan, et al. [15] Lateral oropharyngeal and velar collapse are associated with higher BMIs. This tendency is explained by adipose tissue accumulation in parapharyngeal fat pads. Horner, et al. [10] reported more fat around the retropalatal airspace in more obese subjects. Fat deposits posterolateral to the airspace were observed at the retropalatal but not the retrolingual level. Excessive retropalatal fat accumulation may displace soft tissues (via simple mechanical effects) and narrow the retropalatal airspace, which may thus collapse during sleep. In addition, such fat can compromise upper airway muscle function.
We observed that the increase in DISE grade in the obese group (compared with normal group) was not significant in terms of the retropalatal AP diameter. About this, it is necessary to consider that the incidence of severe obstruction (DISE grade 2) at the retropalatal AP level is high in all BMI groups of OSAS patients. In other words, since the degree of obstruction is already severe, the differences in the degree of airway obstruction between groups with high BMI may not be significant. Additionally, there may also be a threshold at which fat accumulation in certain areas is slowed down, even as BMI increases [15,16]. However, as few patients of BMI ≥30 kg/m2 were enrolled, further research is required.
The absence of significant associations between BMI and obstruction at the retrolingual level can be explained in several ways. Jang, et al. [17] examined the locations and volumes of parapharyngeal fat pads evident in CT scans of OSAS patients with upper airway obstructions, and found that the pads never lay below the level of the uvula. In other words, retropalatal, but not retrolingual, obstruction can be explained by parapharyngeal fat pads. Isono, et al. [16] proposed a box model: at the retrolingual level, changes in the sizes of bony structures such as the mandible, maxilla, and spine (rather than soft tissues) determine airway patency. Additional studies about other factors for retrolingual level obstruction are needed.
Our study is meaningful in that it can help establish therapeutic strategies for obese OSAS patients. Our results provide additional justification for obese patients to lose their weight, as they may help to explain specifically where obesity causes obstruction. In addition, Nadal, et al. [18] found that a higher BMI is associated with lower compliance with continuous positive airway pressure (CPAP) therapy. Therefore, OSAS patients who lose weight would adhere better to CPAP therapy. Our work also supports the efficacy of surgical treatment including lateral pharyngoplasty or uvulopalatopharyngoplasty. So, for obese OSAS patients who cannot tolerate CPAP therapy and consider surgical treatment, this result can be helpful in decision-making.
The limitation of our study is that it is a retrospective study using a specific DISE classification. The classification used in this study has a drawback in that it does not include specific anatomical structures of the tongue, such as macroglossia and lingual tonsils. Considering that various treatments for tongue base, including robotic surgery, are being activated recently, efforts to include it in the DISE classification are needed and more case studies based on this should to be conducted in the future.
We investigated upper airway obstruction status by BMI in Asians. Earlier works have found that obesity increases upper airway obstruction but only Caucasians have been enrolled in such studies [14]. Given the differences in diet, obesity type, and body morphology between Asians and Caucasians, we hypothesized that airway obstruction might differ. However, the trend we observed in our study was similar to Caucasians. A large-scale Asian study on how the BMI affects upper airway obstruction is needed.
Acknowledgements
None
Notes
Author Contribution
Conceptualization: all authors. Data curation: Joo Young Woo, Chang Lok Ji, Tae Kyung Koh. Formal analysis: all authors. Funding acquisition: Soo Kweon Koo. Investigation: Chang Lok Ji, Tae Kyung Koh. Methodology: Joo Young Woo, Soo Kweon Koo. Resources: Tae Kyung Koh. Supervision: Soo Kweon Koo, Chang Lok Ji, Tae Kyung Koh. Validation: Soo Kweon Koo. Visualization: Joo Young Woo. Writing—original draft: Joo Young Woo, Soo Kweon Koo. Writing—review & editing: all authors.