갑상설관낭종에서 에탄올 소작술의 술기
Techniques of Ultrasound-Guided Ethanol Ablation for Thyroglossal Duct Cysts
Article information
Trans Abstract
Thyroglossal duct cyst (TGDC) is the most common congenital neck mass caused by remnants of the thyroglossal duct during fetal development and migration. Traditionally, the Sistrunk procedure has been recommended as the standard surgical treatment for TGDC. However, this procedure is associated with several surgical burdens such as the need for general anesthesia, surgical scarring, and possible postoperative complications. Recently, ultrasoundguided ethanol ablation (US-EA) has been suggested as a good alternative for treating TGDC and has shown excellent treatment outcomes in terms of treatment efficacy and cosmetic satisfaction. Here, we introduce detailed techniques of US-EA for head and neck surgeons to use as a primary treatment of TGDC.
Introduction
Thyroglossal duct cyst (TGDC) is the most common congenital neck mass caused by remnants of the thyroglossal duct during the development and migration of the thyroid in the fetal period [1]. Despite its pathogenesis being associated with embryonic development, clinical signs or symptoms of TGDC are present up to the second decade of life in approximately 50% of all TGDC cases [1,2]. Currently, its treatment of choice is the Sistrunk procedure, which involves the complete excision of the TGDC and a central portion of the hyoid bone [1]. Although the Sistrunk procedure has significantly reduced the recurrence rate after treatment, it is associated with several surgical burdens, including the need for general anesthesia, surgical scarring, and potential postoperative complications such as infection, hematoma, and seroma [3].
Recently, ultrasound-guided ethanol ablation (US-EA) has emerged as a promising alternative for treating TGDC, demonstrating excellent treatment outcomes in terms of treatment efficacy and cosmetic satisfaction while avoiding surgical burdens or major complications [4-7]. This article introduces a detailed technique of US-EA for TGDC to promote its use as a primary treatment of TGDC for head and neck surgeons.
Methods
Pre-procedural assessment
US and CT are the commonly used imaging modalities to evaluate TGDC characteristics. Additionally, US-guided fine-needle aspiration cytology (US-FNAC) is routinely performed to diagnose benign TGDC and to exclude the presence of malignancy. The baseline volume of TGDC is determined by measuring the diameters of the largest axis (a) and the two remaining perpendicular axes (b and c) on US. The volume is calculated using the formula for measuring the volume of an elliptical sphere (V=πabc/6). The volume reduction rate (VRR) is calculated as follows: VRR=(initial volume-final volume)/initial volume×100%. A VRR ≥70% usually indicates treatment success. Cosmetic deformity caused by TGDC is evaluated using the World Health Organization (WHO) cosmetic scoring system (1, no palpable lesion; 2, with palpable lesion but no cosmetic problem; 3, lesion visible only to an experienced physician; and 4, easily visible mass) [8].
Detailed procedure of US-EA
Preparation
US-EA is usually performed in the outpatient setting. The patients are placed in a supine position with their necks extended. After the needle path is planned with US examination, the skin is sterilized with an alcohol swab, similar to the procedure of fine-needle aspiration. Local anesthesia is usually not needed because TGDC does not involve sensory innervation. However, it may be used depending on the surgeon’s or patient’s preference, to reduce pain during needle insertion, particularly when using a large-bore needle.
Based on the TGDC characteristics (particularly, the volume and viscosity of internal contents) and the surgeon’s preference, a 16-25-gauge needle would be used [4,9,10]. A large-bore needle is generally preferred for TGDC with large volumes and/or highly-viscous internal contents [4]. We prefer to use a 21 or 23-gauge needle in usual cases and may use a largerbore needle if necessary.
US–aspiration
For aspiration, the needle is connected to a 10-20 mL syringe held by an aspiration device such as a Cameco syringe holder. The stability of needle manipulation by the operator may be enhanced by interposing an intravenous (IV) extension line between the needle and the syringe. In this case, the operator only manipulates the needle, and an assistant may help aspirate the cystic fluid by manipulating the syringe. In addition, a three-way connector can be used to connect the needle, the IV extension line with the syringe for aspiration, and the ethanol-filled syringe. Through this connection, the syringe for aspiration no longer needs to be replaced with an ethanol-filled syringe when injecting ethanol (Fig. 1).

Needle, intravenous extension line with syringe for cyst aspiration, and ethanol-filled syringe are connected altogether to a three-way connector, which facilitates cyst aspiration and subsequent ethanol injection without the need to change the syringes.
After the needle is inserted into the cyst under real-time US monitoring, the syringe is pulled to aspirate the internal contents (Fig. 2). However, leaving a small number of cystic contents around the needle tip may be helpful to maintain the needle tip within the cyst during the procedure.
US-EA
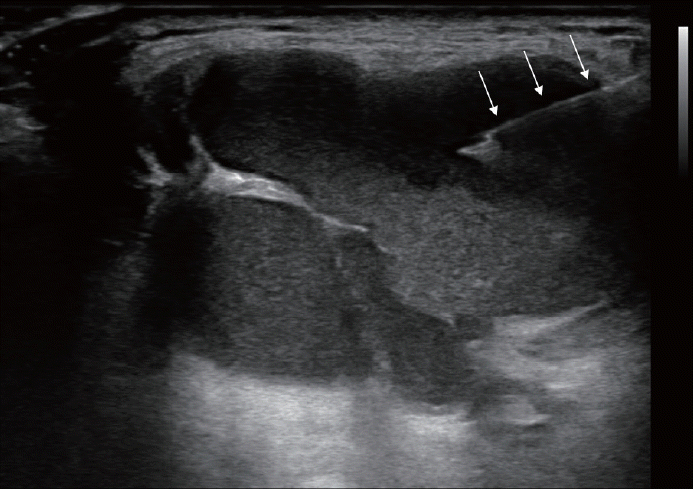
Following the aspiration, 99% sterile ethanol is injected into the cystic cavity using the same needle until the collapsed cyst starts to re-expand (Fig. 3). In our experience, the volume of injected ethanol does not exceed 2.5 mL in most patients. However, if the volume of the aspirate exceeds 20 mL, 5-7 mL of ethanol is injected. Notice that the therapeutic efficacy depends on sufficient contact between the cystic wall and ethanol, rather than the absolute volume of the injected ethanol. Considering that TGDC has no sensory innervation, development of severe pain during ethanol injection indicates that the injected ethanol has leaked out of the cyst, thus, injection should be immediately stopped.

Maintaining the location of the needle (arrows) tip within the cystic cavity, ethanol is injected, and a hyperechoic wave (asterisk) is identified on real-time ultrasonography, representing a ringdown artifact.
After ethanol injection, the needle is maintained in place for 1-2 min to prevent immediate seepage of the injected ethanol and to maximize contact between the cystic wall and the ethanol (Fig. 4). Subsequently, the needle is removed, and the puncture site is lightly compressed for 10 min. Aspiration of injected ethanol is not required.
Post-procedure care
During needle withdrawal, a small amount of injected ethanol would leak from the ablated cyst, causing temporary pain. However, the pain is typically mild, generally lasts for a short duration, and subsides within several minutes [7]. In our previous study involving 28 patients with TGDC treated with EA, 92.9% of patients experienced EA-associated pain, but the pain was mild in approximately 80% of these patients [7]. Some patients may complain of mild dizziness, which is like a drunken sense caused by the absorption of the injected ethanol. However, this phenomenon generally lasts for <10 min, and patients can be settled down for a while, if necessary, until the symptoms subside [7].
Painful swelling of the mass may be present for 3-4 days after the procedure because of the chemical inflammatory reaction induced by the injected ethanol. This inflammation is usually tolerable and is resolved spontaneously. Although there is no recommendation for using antibiotics after EA, we prefer to use antibiotics along with anti-inflammatory drugs to reduce the risk of infection and manage post-procedural pain. In most uneventful cases, patients are followed 1 month after EA.
Results
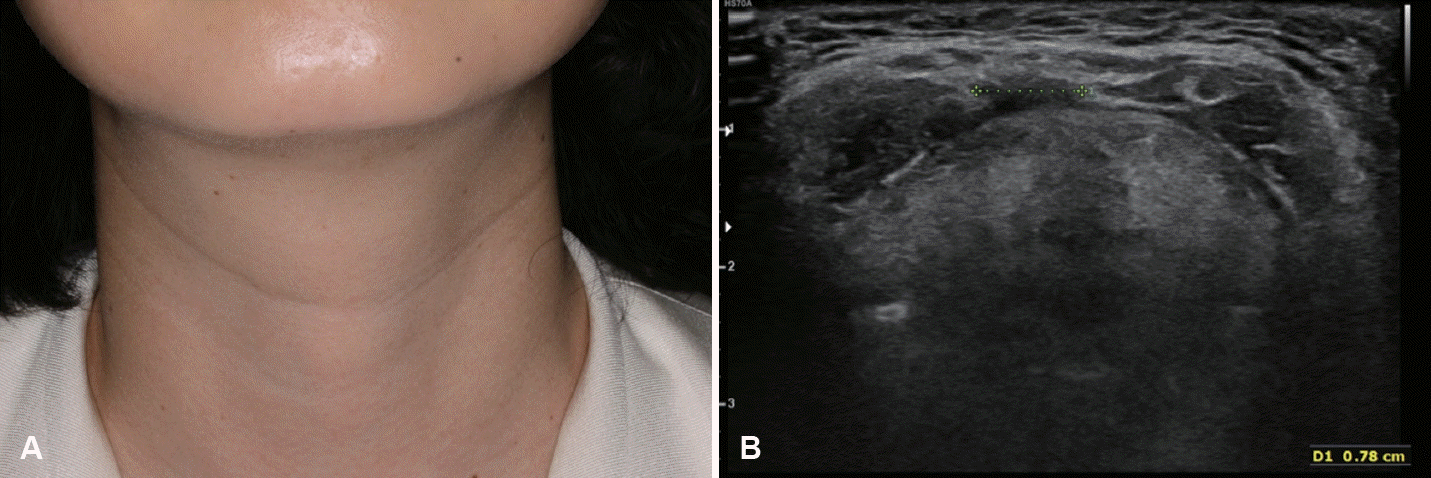
Based on our previous studies involving 28 patients who underwent US-EA for the primary treatment of TGDC between 2017 and 2021, the mean number of EA sessions was 1.4 (range, 1-3), and the median procedure time for each EA session was 6.5 min (range, 5-11 min) [7]. At the last follow-up, the median VRR was 96.2% (58.1%-100.0%). When the treatment success was defined as a VRR ≥70%, the success rate was 96.4% (27/28). Regarding cosmetic outcomes after EA, WHO cosmetic scores decreased to 1 in all patients (Figs. 5 and 6) [7]. No complications were observed other than procedure-related pain [7].
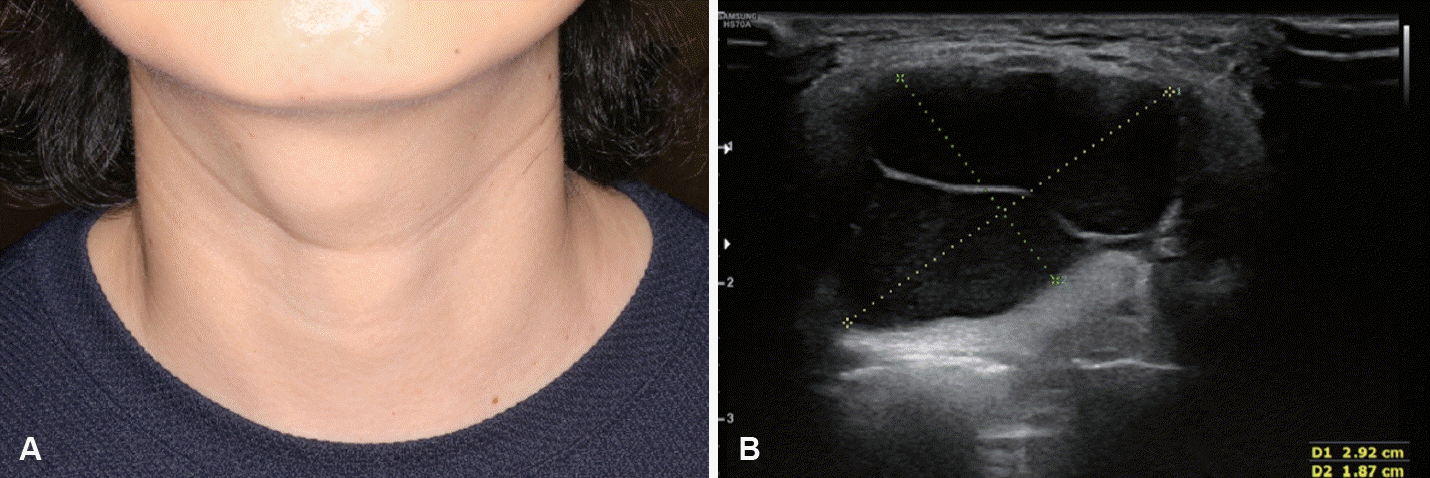
A: Photograph of the external neck with a thyroglossal duct cyst shows an easily visible mass in the anterior neck at the hyoid level. B: This mass is also identified on ultrasonography as a cystic mass with septation.
Discussion
According to 24 studies involving 1374 patients, a painless neck mass is the most common initial presentation of TGDC [1]. Thus, cosmetic deformity caused by TGDC would be the primary reason for receiving treatment from the patient’s perspective [1,7]. Therefore, a surgical treatment that leaves postoperative scars on the anterior neck surface may not be a patient-oriented strategy. Consequently, EA has emerged as a promising nonsurgical alternative for TGDC treatment [5,6].
US-EA is a simple and easily accessible technique for physicians familiar with US-FNAC. Based on our data, all US-EA procedures were successfully performed in the outpatient clinic, with an average procedure time of <10 min without any complications [7]. No major complications were also reported in a recent systematic review of EA for TGDC involving 82 patients from four studies [11]. These findings ensure the technical feasibility and safety of EA for TGDC treatment [11].
Moreover, most studies on EA for TGDC reported highly consistent and stable treatment efficacy, with a 70%-90% VRR and an 80%-90% treatment success rate [4,6,12]. Recently, Park, et al. [5] reported long-term (>2 years) outcomes of EA for TGDC, showing a mean VRR of 81% with a treatment success rate (VRR >50%) of 83%. In our patients who achieved treatment success after EA, no mass regrowth was observed during the median follow-up period of 31 months.
However, when adopting US-EA as a primary treatment for TGDC, several considerations should be introduced. First, although EA demonstrated high feasibility and effectiveness in treating TGDC, it would not be a single-stage treatment, and it may require multiple and longer treatment courses as compared with surgery. Second, we should notice that there is a lack of data that evaluated outcomes of EA in pediatric patients despite that approximately 50% of TGDC presented in pediatric patients. Therefore, we generally do not recommend EA as a first-line treatment for pediatric patients with TGDC.
In conclusion, US-EA is a highly available treatment option for TGDC that can be performed by head and neck surgeons. This technique is effective and accordant with patients’ needs for nonsurgical, noninvasive, and highly cosmetic management. Therefore, we believe that US-EA would be gaining popularity as a primary treatment for benign TGDC.
Acknowledgements
None
Notes
Author Contribution
Conceptualization: Dongbin Ahn. Data curation: Dongbin Ahn, Heungrae Cho. Writing—original draft: Heungrae Cho. Writing—review & editing: Ji Hye Kwak, Minji Oh, Eun Jung Oh, Boseung Jung.