비용종을 동반한 만성비부비동염 환자에서 Dupilumab 치료 시 발생하는 부작용에 대한 후향적 연구
Adverse Events of Dupilumab Injection in Patients With Chronic Rhinosinusitis With Nasal Polyposis
Article information
Trans Abstract
Background and Objectives
Chronic rhinosinusitis with nasal polyposis (CRSwNP) is one phenotype of the diffuse type. In spite of surgical and medical treatment, the polyp recurrence rate is high. Recently biologics are concerned as a therapeutic option of it. To figure out adverse events associated with the dupilumab injection in adult patients with CRSwNP.
Subjects and Method
Retrospective chart review of patients that were treated with dupilumab from January 2022 to December 2022 for CRSwNP was performed.
Results
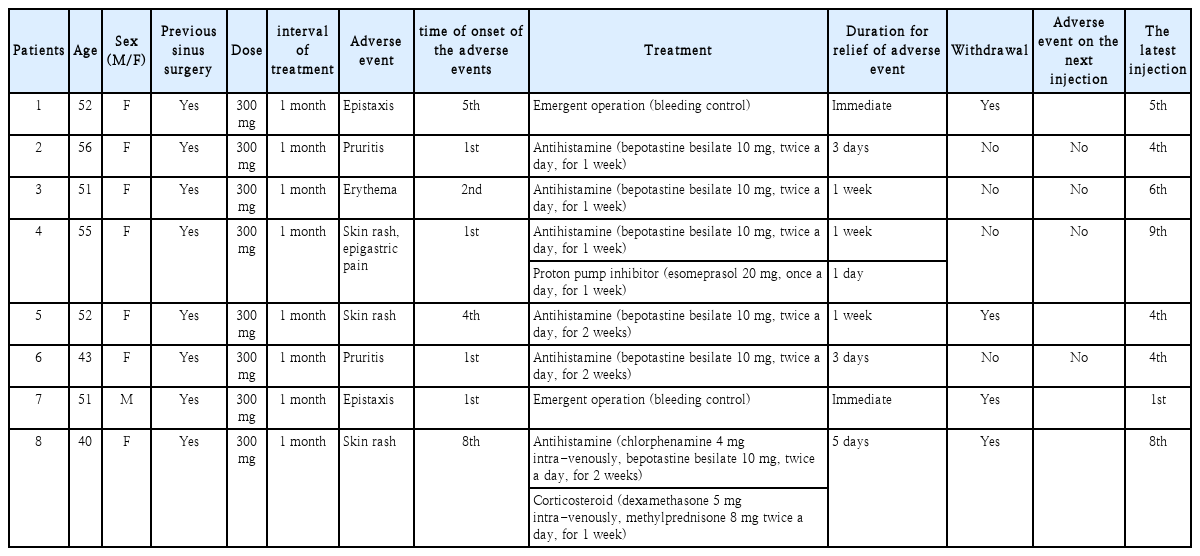
Of 40 patients (23 of male and 17 of female), 8 patients (20.0%) experienced 9 patients (22.5%) adverse events. The most common adverse event was skin reaction (3 patients, 7.5%), followed by 2 of pruritis, 2 of epistaxis. Three patients quit dupilumab after the adverse events, 4 patients continued, and 1 patient hold the next injection.
Conclusion
Dupilumab is reported to be relatively safe medication. However, physician should notice that adverse events including skin rash can occur after dupilumab injection.
Introduction
Chronic rhinosinusitis (CRS) is a common inflammatory disease, with approximately 5%-12% of adults suffering from this disease worldwide [1]. Along the European Position Paper on Rhinosinusitis and Nasal Polyps 2020 (EPOS 2020), primary CRS can be classified along the anatomic distribution (localized or diffuse), endotype dominance (type 2 or non-type 2 inflammation associated). CRS with nasal polyposis (CRSwNP)/eosinophilic CRS, allergic fungal rhinosinusitis and central compartment atopic disease are phenotypes of diffuse type 2 CRS.
In cases of CRS accompanied by nasal polyps, clinical symptoms are severe and treatment is difficult. In most cases, it can be controlled with only classical treatments, such as surgery and steroids; however, nasal polyps recur 20% to 60% of patients within 18 months to 4 years after endoscopic sinus surgery [2], and oral steroids are not suitable for long-term use because of side effects.
Biological agents are medications that are isolated from organisms. Since the past two decades, biologics have been approved for use in chronic inflammatory diseases, such as eosinophilic asthma, atopic dermatitis, and chronic urticaria. Additionally, several biologics, such as the anti-interleukin (IL)-4 receptor α monoclonal antibody dupilumab, anti-immunoglobulin E (IgE) monoclonal antibody omalizumab, and anti-IL-5 monoclonal antibody mepolizumab have been approved by the US Food and Drug Administration (FDA).
Dupilumab is a monoclonal antibody that binds to the IL-4 receptor α subunit to inhibit IL-4 and IL-13, which are the central drivers of type 2 inflammation [3]. Thus, inhibition of these signaling pathways by the two ILs can prevent the recurrence of polyps. It was the first biologic approved by the US FDA in June 2019 for adult patients with uncontrolled CRSwNP. It is known to be safe without critical side effects. It can cause injection site rashes, myalgia, gastritis, and other mild complications.
Herein, we performed a retrospective study for the patients who took the dupilumab-injection at the single center to review the adverse events associated with the dupilumab.
Subjects and Methods
Inclusion and exclusion criteria
We perform a retrospective chart review of patients receiving dupilumab-injection for CRSwNP at the Department of Otorhinolaryngology-Head and Neck Surgery of Kosin University Gospel Hospital from January 2022 to December 2022. 300 mg of dupilumab was administered subcutaneously for each time.
Data collection, variables, and study outcomes
Retrospective charts review was performed for enrolled patients. Demographic information including age and sex was collected. The presence of underlying disease (e.g. asthma, atopic dermatitis) were included.
Visual analogue score (VAS, range of 0 to 10) for nasal symptoms including nasal obstruction, rhinorrhea and hyposmia, change in endoscopic nasal polyp score (NPS, range of 0 to 8; higher scores indicate higher polyp burden) and identification score of Korean Version of the Sniffin’ Stick II (KVSS II) test involved 16 odors familiar to Koreans were collected before each times of injection for clinical outcomes. And serum eosinophil counts and tissue eosinophil counts if patients got the sinus surgery were collected.
Any adverse events after dupilumab injection was recorded. For these patients, the time of onset the adverse event, whether the dupilumab was discontinued thereafter were also recorded.
Statistical analysis
Descriptive statistical analysis, Wilcoxon signed-rank test and Mann-Whitney U test were performed using SPSS 25.0 (IBM Corp., Armonk, NY, USA). For the assessment of the risk factors associated with the development of adverse events, qualitative variables were compared using the chi-square test (or Fisher’s exact test when necessary). Student’s t-test was used to compare the distribution of quantitative data (or Mann-Whitney’s test when distribution departed from normality or when homoscedasticity was rejected). p-value under 0.05 is considered to indicate statistical significance.
Ethics
Institutional Review Board (IRB) of Kosin University Gospel Hospital approval was obtained for the review and analysis of this data. This study was a retrospective cohort study and no consent was required for this study (IRB fine number: KUGH 2023-04-033).
Results
Patient demographics
Fourty patients (22 of male and 18 of female) were enrolled for this study. Mean age was 49.5 (range of 24 to 83, SD=12.43). Sixteen (40.0%) patients took the sinus surgery more than twice before the first dupilumab treatment. Mean times of administration was 4.23 (range of 2 to 10). Six patients (15.0%) have history of asthma. There was no patient with the other type 2 inflammatory disease like atopic dermatitis.
EPOS 2020 guideline suggested indications for biological treatment in CRSwNP as 5 criteria and cut-off points [4]. Thirty eight patients (95.0%) had evidence of type 2 inflammation (tissue eosinophil ≥10/high power field, odds ratio (OR) blood eosinophil ≥0.25×109/L, OR total IgE ≥100) 24 patients (60.0%) met three or more of five criteria. In detail, 38 patients (95.0%) showed tissue eosinophil ≥10/high power field on biopsy, 14 patients (35.0%) showed total IgE ≥100/μL and 24 patients (60.0%) showed blood eosinophil ≥0.25×109/L. On the other hand, EPOS revised guidance in the management of patients with CRS in collaboration with European Forum for Research and Education in Allergy and Airway Diseases [5]. This update suggested lowering the cut-off point of blood eosinophil counts for the evidence of type 2 inflammation from 0.25×109/L to 0.15×109/L. Along this update, 27 patients (37.5%) met this cutoff point, but there was no change on number of indication criteria met. Details about demographics is summarized in Table 1.
Adverse events
Eight of 40 patients (20.0%) experienced nine adverse events after dupilumab administration. Most patients were female (n=7, 87.5%) and mean age was 50 (range of 40 to 56, SD=5.61). Mean time of onset of adverse event was 2.89 (range of 1 to 8). Four of adverse events onset at least after second administration. Six patients (75% of patients with adverse events) experienced the skin reaction, two patients experienced epistaxis, and one patient experienced pruritis without skin rash. And four of patients with adverse event discontinued the dupilumab treatment according to the physician’s decision. Two cases of epistaxis are probably caused by previous sinus surgery. Excluding those two cases, two patients discontinued dupilumab due to the adverse event (Table 2). On the other hand, there was no case of hypereosinophilia (blood eosinophil ≥1.5×109/L) on this study. Figures of adverse events of patient number 4, 5 and 8 are shown in Fig. 1.
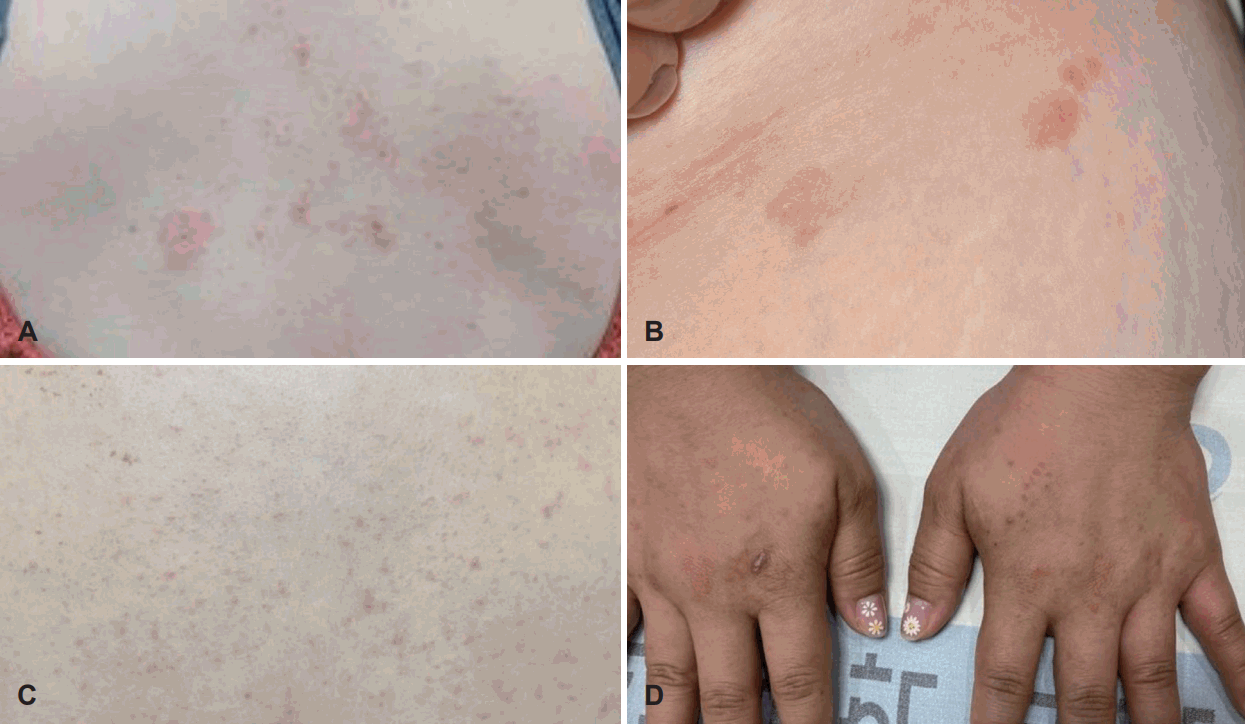
Skin rashes occurred after the dupilumab injection of patients number 4, 5 and 8 of the Table 1 respectevely. A: Multiple pruritic erythematous papules and a few macules are visible on the back of the patient number 4. B: A few pruritic erythematous maculopapular lesions are visible on the inguinal area of the patient number 5. C and D: Multiple localized pruritic erythematous papules and patches on trunk and hands were shown.
Clinical outcomes
We analyzed the changes of clinical variables between before the first injection and the last injection using Wilcoxon signed-rank test. For entire enrolled patients, all three clinical variables improved with statistically significance; VAS improved from 6.95 to 3.50 (p<0.005), identification score of KVSS II improved from 7.30 to 9.20 (p<0.005), and NPS improved from 4.13 to 2.53 (p<0.005). For patients with adverse event, all three clinical variables improved, but only VAS has clinical significance; VAS improved from 7.75 to 3.63 (p=0.011), identification score of KVSS II improved from 6.75 to 8.12 (p=0.206) and NPS improved from 4.13 to 3.00 (p=0.137). Otherwise, all clinical variables of patients with adverse events didn’t show statistically significant differences (p>0.05) on Mann-Whitney U test compared to those of patients without adverse events.
Discussion
Dupilumab is a humanized IgG4 monoclonal antibody targeting the IL-4 receptor alpha chain that inhibits the signaling of two cytokines, IL-4 and IL-13. These two ILs are among the signaling drivers of type 2 inflammation. In particular, IL-4 induces Th0 cells to switch to Th2 cells, and Th2 cells promote IL-4 and IL-13 production. IL-4 and IL-13 contribute to skin barrier dysfunction, the itch-scratch cycle, and increased susceptibility to a skin infection [6].
IL-4 and IL-13 drive type 2 inflammation, which plays a crucial role in atopic dermatitis, asthma, CRSwNP, and eosinophilic esophagitis. Dual inhibition of both IL-4 and IL-13 contributes to the reduction of type 2 inflammation; therefore, they can migrate to the symptoms of these diseases. First, it has been approved for patients with atopic dermatitis. In 2019, the US FDA approved a dupilumab injection to treat adults with CRSwNP.
The efficacy and safety of dupilumab in patients with CRSwNP have been reported in two large multicenter randomized controlled trials, LIBERTY NP SINUS-24 and SINUS-52 [7]. Total of 276 patients were enrolled in the SINUS-24 clinical study: 143 received dupilumab every 2 weeks for 24 weeks and 133 received a placebo. In the SINUS-52 clinical trial, 448 patients were enrolled; 150 patients were administered dupilumab every 2 weeks for 52 weeks, while 145 patients were treated every 2 weeks for 24 weeks, followed by 4 weeks for a total of 52 weeks, and the remaining 153 patients received a placebo. Compared to the placebo group, the dupilumab group showed significantly better results in nasal polyp size, Lund-Mackey endoscopic score, nasal obstruction, congestion, and sense of smell, reducing the need for additional systemic steroid use or surgical treatment.
In addition, dupilumab is known to be effective without life-threatening side effects. In the SINUS-24 and SINUS-52 studies, the most common side effect after dupilumab administration was an injection site rash [7]. Additionally, nasopharyngitis, myalgia, conjunctivitis, gastritis, insomnia, and headache have been reported. Side effects, such as nasopharyngitis, asthma exacerbation, headache, and epistaxis, were more common in the placebo group than those in the dupilumab group.
Additionally, these side effects are known to occur similarly in studies of patients with moderate-to-severe atopic dermatitis and moderate-to-severe asthma, which are indications of dupilumab administration. Injection site reaction was the most common side effect among all patient groups for indications of dupilumab administration (moderate to severe asthma, moderate to severe atopic dermatitis, eosinophilic esophagitis, prurigo nodularis, and CRSwNP) and some non-fatal side effects, such as upper respiratory tract manifestations (infection, oropharyngeal pain bronchitis) in patients with moderate to severe asthma or eosinophilic esophagitis, oral herpes, and ocular complications (eye pruritus, blepharitis, and conjunctivitis) in adults with moderate to severe atopic dermatitis.
However, in a randomized controlled trial, there were no reported cases of skin rashes occurring in areas other than the injection site. To the best of our knowledge, only a few cases have reported skin rashes on the head and neck area in patients with atopic dermatitis [8]. Just few cases has been reported in patients with CRSwNP [9].
The skin rash, which occurred after dupilumab administration in three of our patients, was an urticarial rash, representing a type II allergic reaction. It was relieved within a few days with or without antihistamines. However, no antigens are known to be sensitized. Considering no other systemic medication was administered to the patients around the dupilumab injection, dupilumab or some additive in prefilled medication could cause an immune response. Other unpredictable antigens can also cause such a reaction; however, this was not observed in our investigation.
In cases of dupilumab therapy on patients with atopic dermatitis, there is a hypothesis that inhibition of IL-4Ra can activate T-helper-1 (Th1)/Th17 mediated immune cascades on skin. And Th1 mediated immune response can cause psoriasiform dermatitis [9]. CRSwNP is also the classic type 2 inflammation, and inhibition of this Th2 mediated immune cascade can also cause Th1 mediated immune cascade.
However, in this study, not all skin reactions except one case were like psoriasiform dermatitis. Others were maculopapular rash. This shows that there could be some other mechanism of dupilumab induced skin reactions.
There is little known about a predictive factor of dupilumab associated adverse events. In case patient with the atopic dermatitis, ocular adverse event is reported to be correlated with IL-33 concentration [10]. However, there is no reported factor to predict the occurrence of the adverse event in patients with CRSwNP. In this study, only the female sex is associated with occurrence of the skin rash. More experience and study may be required to figure out the predictive factors of dupilumab associated adverse events in patients with uncontrolled CRSwNP.
There are no definite guidelines for the management of skin rash or deciding whether and when to restart dupilumab administration. In our cases, the dupilumab injection was reinitiated after the first onset of skin rash because all rashes were revealed within a few days and were not accompanied by any critical complications, such as anaphylaxis. In the following administration, two of these patients no longer complained of rash. In contrast, one patient complained of a rash with similar characteristics on the same site. For this patient, we administered additional dupilumab, with her symptoms controlled by other medications.
There are no definite reasons that revealed that the rashes did or did not recur, and there is no way to predict whether a skin rash will recur. The physician must decide how to manage the complications clinically and whether to continue the treatment. In these situations, we have to consider whether the symptoms are tolerable, the critical complication is present, and whether continuing the treatment is more beneficial than harmful to the patient.
In spite of the presence of the adverse event, clinical variables of patients improved with statistical significance through dupilumab treatment. When compared to patients without adverse events, there were no statistically significant differences in changes of clinical variables. These two results indicate that the presence of adverse events doesn’t affect the effectiveness of dupilumab. Therefore, continuing dupilumab therapy would be beneficial to improvement of nasal symptoms of patients if the safety of patient is secured.
Except for two cases of epistaxis, most of the complications were skin reactions including itching sensation and rash. Both cases of epistaxis had to get an emergent operation for bleeding control. And similar intraoperative findings; diffuse oozing rather than definite arterial bleeding on a single bleeding focus were shown on both patients. And epistaxis occurs one month after the surgery on one patient. Because of these two aspects, it is confusing that two cases of epistaxis are actually the postoperative complication or dupilumab-induced.
In conclusion, dupilumab is proposed as an effective drug for uncontrolled CRSWNP, which is relatively safe and has few side effects. However, in our experience, skin-related adverse events are relatively common and there may be unexpected adverse events. Therefore, more experience and research should be performed.
Acknowledgements
None
Notes
Author contributions
Conceptualization: Jaehwan Kwon, Jooyeon Kim. Data curation: Pooreum Kang, Donggyu Choi. Formal analysis: Pooreum Kang. Methodology: Donggyu Choi. Supervision: Jooyeon Kim. Validation: Jooyeon Kim. Visualization: Changhoi Kim. Writing—original draft: Pooreum Kang. Writing—review & editing: Jaehwan Kwon.