 |
 |
AbstractMyoepithelioma is a rare tumor of the salivary glands. Occurrence of myoepithelioma in the nasal cavity is even rarer, and there has been no case wherein the change in the size of the tumor was confirmed by imaging. In this report, a 84-year-old female was found to have a rapidly enlarged tumor on the preoperative image, but she had shown no significant size change in a medical examination six months ago or in the paranasal sinuses view three months ago. The image showed a rapid increase in the size of the cystic component, so the endoscopic medial maxillectomy was performed. There was no recurrence of the tumor at follow-up six months after surgery. Although myoepithelioma is rarely found in the maxillary sinus of the sinonasal cavity, it is important to suspect myoepithelioma and consult a pathologist if it shows a similar pattern to the current case.
IntroductionMyoepithelioma is a slow-growing benign tumor that originates from the salivary glands and accounts for 1% of all salivary gland neoplasms [1]. Myoepithelioma was first reported by Sheldon [2] in 1943. It was considered a variant of pleomorphic adenoma in the 1940s because of their similar components and occasional malignant potential, but the World Health Organization (WHO) has now classified myoepithelioma as an individual disease [3]. The salivary glands are the most common site of myoepithelioma, but it can also occur in other sites such as the external auditory canal, orbit, and nasal cavity [4]. Given that diagnosis is difficult even for pathologists because of diverse histomorphological and immunohistochemical characteristics, it is important to keep in mind the possibility of myoepithelioma. The occurrence of myoepithelioma in the sinonasal cavity is rare, and only 15 cases have been reported thus far [4]. Furthermore, it is not easy to detect the occurrence of myoepithelial tumors in the sinonasal cavity. The growth characteristics of sinonasal myoepithelioma through imaging studies have not been reported yet. In the current paper, we retrospectively report the case of an 84-year-old female who underwent endoscopic resection, which showed the rapid growth of the cystic component of a tumor. This is the first report of a myoepithelioma in the maxillary sinus of the sinonasal cavity in Korea.
CaseAn 84-year-old female with hypertension, stenotic coronary artery disease, right cerebral infarction visited the outpatient clinic because of left facial swelling without tenderness and left epiphora that had occurred 1 month ago. Coincidentally, she had brain MRI for health check-up six months ago, and there was an unreported 2.7 cm-sized cystic and solid mass in the left maxillary sinus abutting the medial wall of left maxillary sinus (Fig. 1A and B). This mass probably originated from the medial wall of the left maxillary sinus. After visiting the internal medicine clinic for a postnasal drip 3 months ago, there was a round mass-like haziness of left maxillary sinus on paranasal sinus (PNS) view (Fig. 1C). It grew slowly, but no rapid growth of size compared to the previous Brain MRI 3 months ago. She did not complain of facial swelling or epiphora at the time. She underwent PNS CT and neck MRI, which revealed a well-defined cystic and solid mass that is approximately 3.8 cm in size filling the left maxillary sinus. MRI showed that the mass had solid portions at the medial and lateral aspects, which showed relatively low T2 signal intensity with heterogeneous contrast enhancement (Fig. 2). The cystic portion of the mass showed internal septations with marginal contrast enhancement in a T1-weighted gadolinium-enhanced image. CT showed soft tissue density in the left nasal cavity and maxillary sinus. The center of mass has low density without enhancement by a contrast medium, but the outside is enhanced. It means that the center of mass has a cystic component and the outside has a soft tissue component (Fig. 2). The bony remodeling and erosion of the posterior wall of the left maxillary sinus was demonstrated by CT. In the retrospective view, the cystic portion of the tumor shown by the MRI increased within three months of the interval. The patient underwent septoplasty for correction of septal deviation to left side, and endoscopic medial maxillectomy with a safety margin. Endoscopic medial maxillectomy was performed including nasolacrimal duct, anterior stump of inferior turbinate, medial wall of maxillary sinus.
Histological examination showed plasmacytoid myoepithelial cells with cords and sheets growth patterns in fibromyxoid stroma without ductal differentiation, nuclear atypia or mitosis (Fig. 3A and B). Immunohistochemically, the labeling index of Ki-67 positive cells was about 7%. The cellular area near the cystic portion showed a relatively high Ki-67 labeling index (Fig. 3C). The tumor cells were partly positive for smooth muscle actin (SMA), and glial fibrillary acidic protein (GFAP) and diffusely positive for the p40, p63, and S-100 protein. (Fig. 3D). The histologic findings were consistent with myoepithelioma. The patient was followed up for six months after surgery, and there were no suspicious findings of tumor recurrence (Fig. 4).
The Yongin Severance Hospital IRB approved wavier of consent for this study (IRB No. 9-2022-0002).
DiscussionMyoepithelioma is a tumor that originates from myoepithelial cells. These cells are mainly seen in the intercalated ducts and acini of the salivary glands. Myoepithelioma is usually a painless, slow-growing, benign tumor. However, it can sometimes be locally aggressive. Myoepithelioma mainly occurs in the parotid gland compared with other salivary glands [5]. In Korea, so far, a myoepithelioma in the sinonasal cavity has been reported only in the nasal septum and nasal cavity, and no cases in the maxillary sinus have been reported yet [4,6,7]. In this case, a myoepithelioma of maxillary sinus origin was reported for the first time in Korea.
The WHO suggests that a diagnosis of myoepithelioma should be made if the neoplasm contains less than 5% ductal components [8]. Myoepithelioma is composed of myoepithelial cells with solid, reticular, or myxoid patterns of growth. The cells are classified as spindle, epithelioid, plasmacytoid, and clear cell types [8]. The growth pattern and cell type do not correlate with the prognosis of the disease [9]. In this case, sheets of homogenous, large plasmacytoid cells, prominent eosinophilic cytoplasm and fibromyxoid stroma was seen on hematoxylin and eosin (H&E) staining (Fig. 3A), so pleomorphic adenoma and myoepithelioma can be considered. However, it was closer to myoepithelioma because of the absence of ductal differentiation.
As known as myoepithelial cells which exhibit a wide range of differentiation, the myoepithelial tumor cells are positive for a variable epithelial and mesenchymal immunohistochemical markers. This tumor stains positive for cytokeratins (AE1/AE3, CK14, and CK5/6), S-100 protein, p63, p40, vimentin, calponin, GFAP, SMA, and carcinoembryonic antigen [10]. Immunohistochemically, the S-100 protein is the main marker for myoepithelioma [1], and a diagnosis will rarely be made if this stain is negative [11]. In our case, tumor cells were partly positive for SMA and GFAP and diffusely positive for the p40, p63 and S-100 protein.
Ki-67 index means cell proliferative activity [12]. So, the Ki-67 marker is useful for diagnosing malignant myoepithelioma. In this case, the Ki-67 labeling index was low, about 7%. In addition, considering the absence of abnormal mitosis, vascular invasion, and cellular polymorphism on H&E, it was diagnosed as benign myoepithelialoma. Although it was not malignant, it showed rapid growth of the cystic component within 3 months. The reason is thought to be because most of the Ki-67 staining, which indicates cellular proliferation, is seen in the cystic component and the myoepithelial cells are densely located around it (Fig. 3C). Above-average Ki index, 7% also seems to have contributed to the faster growth than in other benign myoepitheliomas ranged from 0.9%-9.1% (mean: 5.4%; standard deviation: 3.1%) [9]. Therefore, we think that high cell proliferative activity and the high cellularity of the cystic portion as indicated by the Ki-67 staining were the reason for the rapid growth of the tumor. And it seems that the secretions of these cells have accumulated inside the rapidly proliferating tissue. In the future, the tumor seemed likely to progress to malignancy.
Macroscopically, myoepitheliomas are usually well-encapsulated tumors; therefore, complete excision is important. The treatment choice for myoepithelioma is generally complete surgical excision [1]. In the current case, the endoscopic wide excision of the tumor was performed with clear margins. No recurrence was observed during the six-month follow-up period without additional treatment.
Benign myoepithelioma is usually an asymptomatic mass that slowly increases in size over several months or years. It is known that malignant myoepithelioma increases in size, but there has been no confirmation of the growth rate from imaging evidence in papers reporting benign myoepithelioma. In the current case, cystic components and solid components were mixed.
The maxillary sinus tumor in MRI and CT six months before the visit was not growing significantly in PNS view three months later. In the next three months, the cystic component expanded rapidly, showing bone remodeling and facial swelling caused by the mass effect. Therefore, most myoepithelioma is slow growing, but in the case of myoepithelioma, where cystic components are mixed, the possibility of rapid growth should be kept in mind.
In other words, if a clinician or pathologist does not consider the possibility of myoepithelioma, an appropriate immunohistochemical test may not be performed, and diagnosis may be difficult. Therefore, although the occurrence of myoepithelioma in the sinonasal cavity is rare, it is important to suspect myoepithelioma and consult a pathologist if it shows a similar pattern to the current case.
ACKNOWLEDGMENTSThis study was supported by a faculty research grant of Yonsei University College of Medicine for 6-2020-0118.
NotesAuthor Contribution Conceptualization: Jong-Gyun Ha. Data curation: Jang Won Oh. Writing—original draft: Jang Won Oh. Writing—review & editing: Chae Jung Park, Yoon Jung Choi, Jong-Gyun Ha. Fig. 1.Health check up images. A and B: Brain MRI images as a health check-up six months before the first visit. Axial (A) and coronal (B) T1-weighted gadolinium-enhanced images showing that the contrast-enhanced part on the outside and the cystic part with a low signal on the inside were observed. C: Paranasal sinus view image three months before the first visit. There was no significant growth in size compared to brain MRI three months ago. 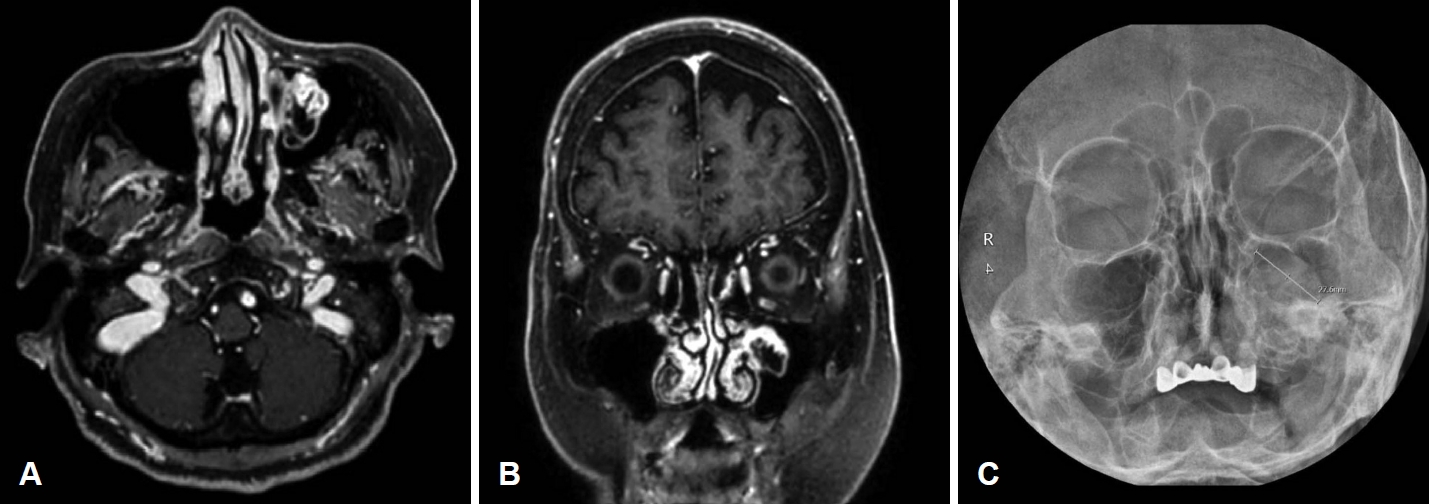
Fig. 2.Preoperative MRI and CT images. A and B: Coronal (A) and axial (B) planes of CT demonstrating heterogeneous contrast enhancement by the tumor. The bony remodeling and erosion of the posterior wall of the left maxillary sinus was demonstrated at CT. C: Axial T1-weighted image without gadolinium enhancement showing a hypointense lesion occupying the left nasal cavity. D: Axial T2-weighted image showing solid portions at the medial and lateral aspects of the lesion showing relatively low T2-signal intensity and cystic portions in the center of the lesion showing high T2 signal intensity. E and F: Axial (E) and coronal (F) T1-weighted gadolinium-enhanced image showing a low-intensity signal was observed in the center of the lesion, and contrast enhancement was observed in the outer part. 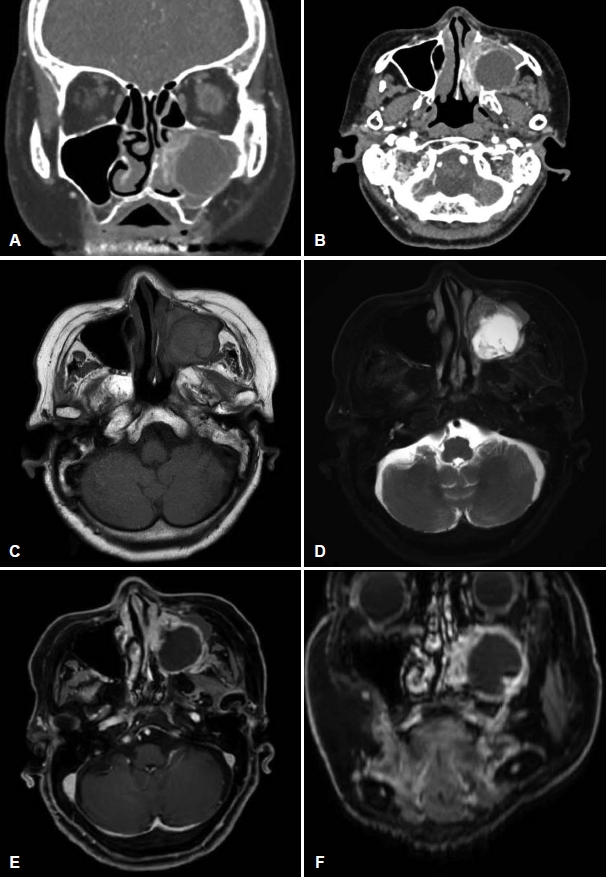
Fig. 3.Microscopic view. A and B: Histologic findings showing cords or sheets of typical plasmacytoid myoepithelial cells among fibromyxoid stroma without mitosis or cytological atypia (hematoxylin and eosin staining; A, ×100; B, ×200). C and D: Tumor cells were positive stainning with the cellular area around the cystic portion for Ki-67 labeling index (C), about 7%, and diffusely positive for the S-100 protein (D) (immunohistochemical studies; C, ×100; D, ×200). Arrow, plasmacytoid myoepithelial cell: these cells have bright eosinophilic with eccentric nuclei; arrowhead, hyalinized stroma. 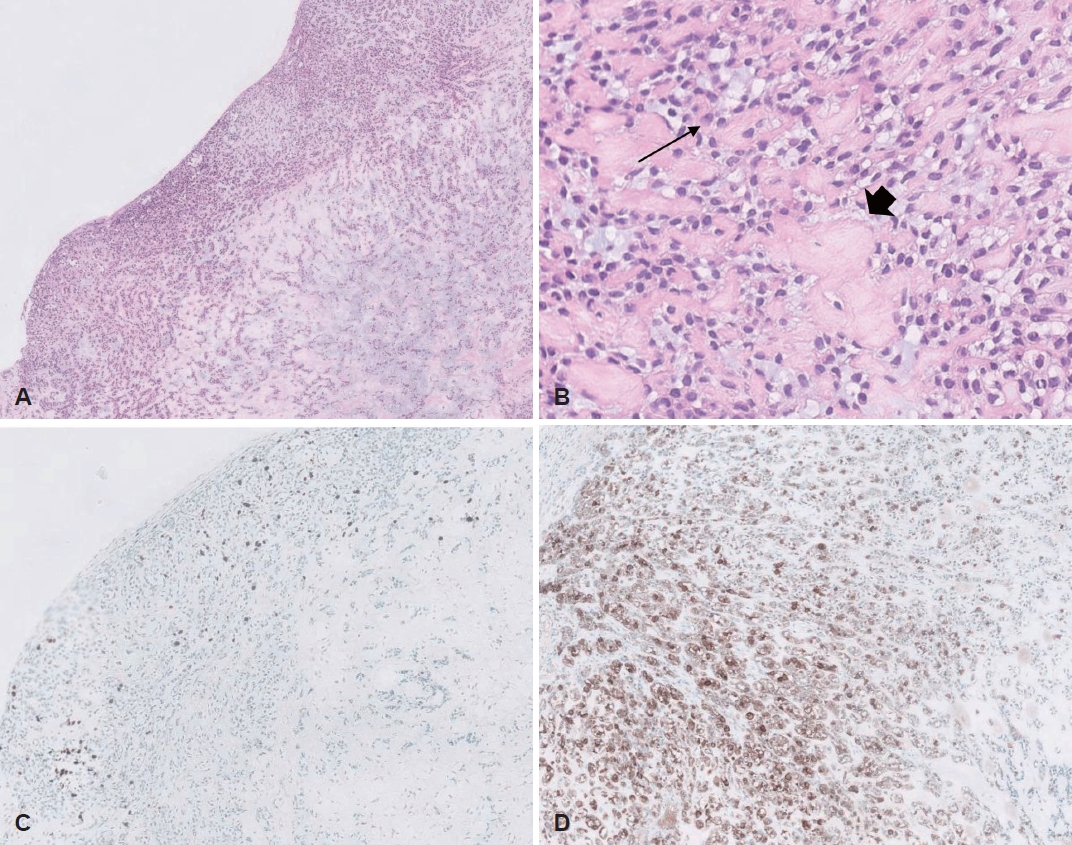
Fig. 4.Nasal endoscopic images. A: Nasal endoscopic view before surgery. Bulging of the medial wall of the left maxillary sinus was observed. B: Intraoperative nasal endoscopic view. After left middle meatal antrostomy, cystic portion of the tumor was observed in the medial wall of maxillary sinus, the mucosal surface of lesion was smooth and the inside was filled with fluid. C: Postoperative nasal endoscopic view six months after surgery. There were no suspicious findings of tumor recurrence. Asterisk, cystic portion of the mass; MT, middle turbinate; IT, inferior turbinate. 
REFERENCES1. Sciubba JJ, Brannon RB. Myoepithelioma of salivary glands: Report of 23 cases. Cancer 1982;49(3):562-72.
2. Sheldon WH. So-called mixed tumors of the salivary glands. Arch Pathol 1943;35:1-20.
3. Barnes L, Eveson JW, Reichart P, Sidransky D. Pathology and genetics of head and neck tumours. World Health Organization Classification of Tumours.. 1st ed. Lyon: IARC; 2005. p. 259-60.
4. Kang JW, Im SK, Song CE, Jung SY. A nasal myoepithelioma removed through preoperative embolization and endoscopic surgery: A case report and literature review. Korean J Otorhinolaryngol-Head Neck Surg 2019;62(12):747-54.
5. Ellis GL, Auclair PL. Tumors of the salivary glands. Atlas of tumor pathology 3rd series.. Arlington, TX: Amer Registry of Pathology; 1996.
7. Moon JY, Jeong JY, Kim JS, Heo SJ. A case of myoepithelioma involving nasal septum. J Clin Otolaryngol Head Neck Sur 2020;31(2):234-7.
8. Simpson RH, Jones H, Beasley P. Benign myoepithelioma of the salivary glands: A true entity? Histopathology 1995;27(1):1-9.
9. Nagao T, Sugano I, Ishida Y, Tajima Y, Matsuzaki O, Konno A, et al. Salivary gland malignant myoepithelioma: A clinicopathologic and immunohistochemical study of ten cases. Cancer 1998;83(7):1292-9.
10. Gore CR, Panicker N, Chandanwale S, Singh BK. Myoepithelioma of minor salivary glands - A diagnostic challenge: Report of three cases with varied histomorphology. J Oral Maxillofac Pathol 2013;17(2):257-60.
|
|
||||||||||||||||||||||||||||||||||||||||||||

 |
 |