Introduction
Epithelial-myoepithelial carcinoma (EMCa) is rare, accounting for only 1% of all salivary gland tumors [1,2]. Despite its benign histological features, local recurrences occur in approximately 23%-50% but distant metastases occur in 2.6% of all patients [2-5]. Distant metastasis shortens the survival period of patients with EMCa [6]. However, the treatment guidelines for distant metastasis remain to be established. Moreover, only 26 cases of metastatic salivary gland EMCa have been reported to date, with none reported in Korea [7]. We report a case of a parotid gland EMCa with lung metastasis, treated with adjuvant chemotherapy after parotidectomy and radiotherapy. Further, we review the treatment strategies and outcomes of 26 global cases of EMCa with distant metastasis.
Case
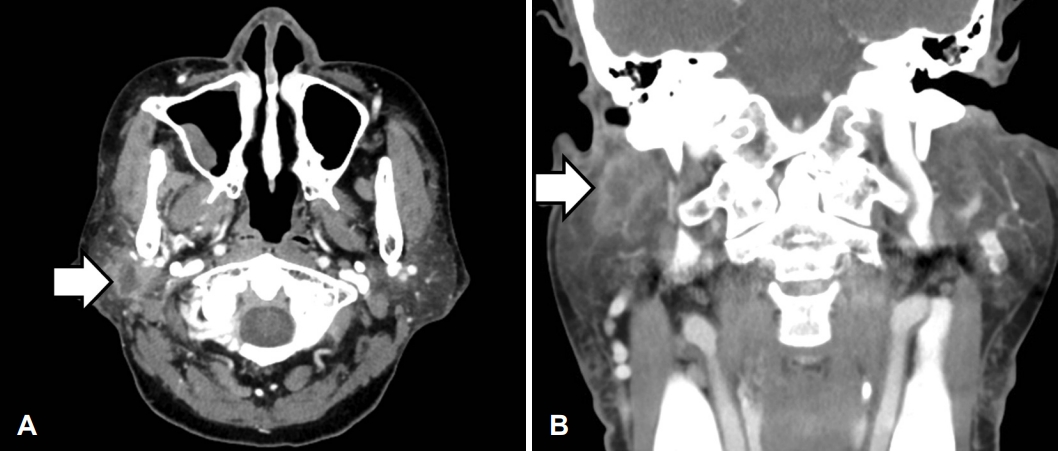
A 68-year-old female visited our outpatient clinic on March 30, 2018, with a 1-week history of right parotid gland swelling. We noted a firm 2.5-cm mass at the right parotid area. Neck contrast-enhanced CT revealed an enhanced 1.8-cm mass with irregular surface and necrosis, connected to the deep and superficial parotid gland lobes; cervical lymph node enlargement was absent (Fig. 1). After 2 weeks, she had newonset House-Brackman (H-B) grade IV facial nerve palsy lasting 1 month preoperatively. A core needle biopsy revealed a few atypical tubular and glandular structures in the desmoplastic stroma, suggestive of a salivary gland epithelial myoepithelial tumor; as cancer was not confirmed, neck dissection was not considered.
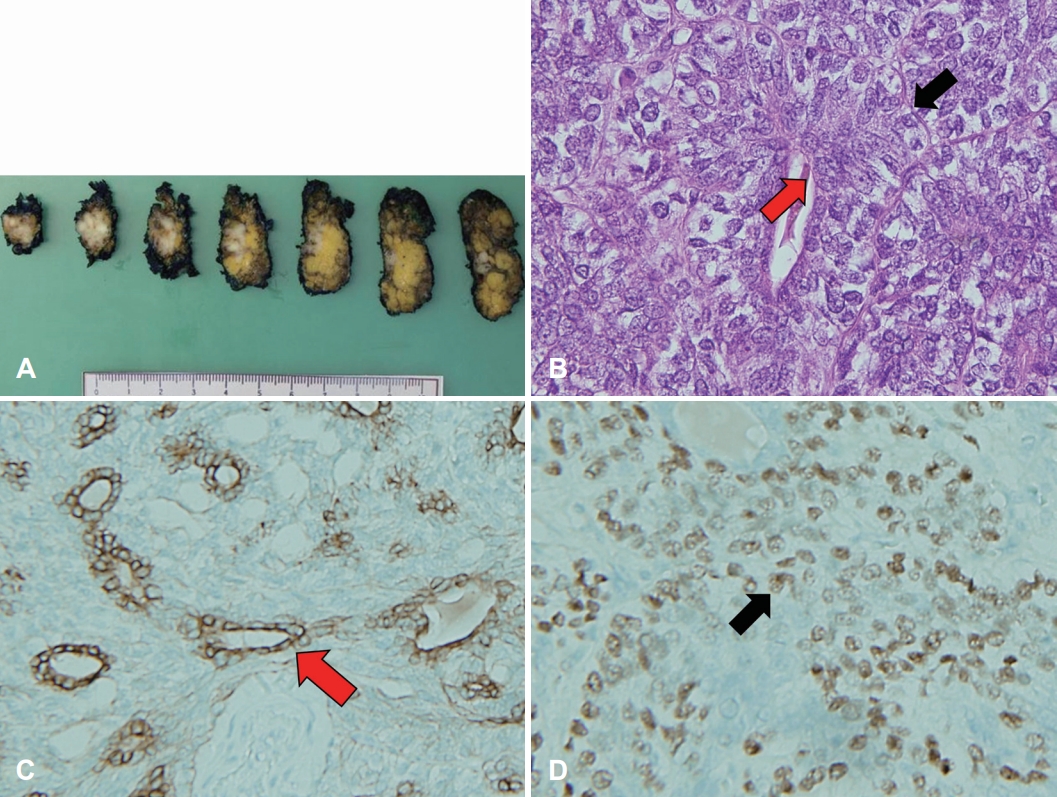
On May 16, 2018, she underwent right total parotidectomy and peritumoral lymph node resection with facial nerve preservation as facial nerve invasion was absent. The resected tumor was a 1.6×0.8×2.4-cm, gray, solid, multinodular mass. Microscopically, it showed a biphasic pattern of epithelial cells and myoepithelial cells. Myoepithelial anaplasia, nuclear atypia, and tumor-free resection margins were noted. There was no gross extraparenchymal extension, microscopic perineural tumor invasion, angiolymphatic invasion, or necrosis. On immunohistochemical analysis, the epithelial cells showed positive for pan-cytokeratin (PAN-CK), and epithelial membrane antigen (EMA). Myoepithelial cells showed positive for P63, smooth muscle antibody (SMA), and vimentin, confirming the diagnosis of EMCa (Fig. 2). There was no metastasis detected in resected lymph nodes.
PET-CT, abdominal ultrasonography, gastroesophageal endoscopy, and chest CT revealed no evidence of distant metastasis. Facial nerve palsy improved to H-B grade II within 2 postoperative days, with no further improvement. From June 11 to July 27, 2018, the patient underwent 33 sessions of postoperative radiotherapy (PORT), with a total dose of 66 Gy, due to a high risk of local recurrence and facial nerve palsy history.
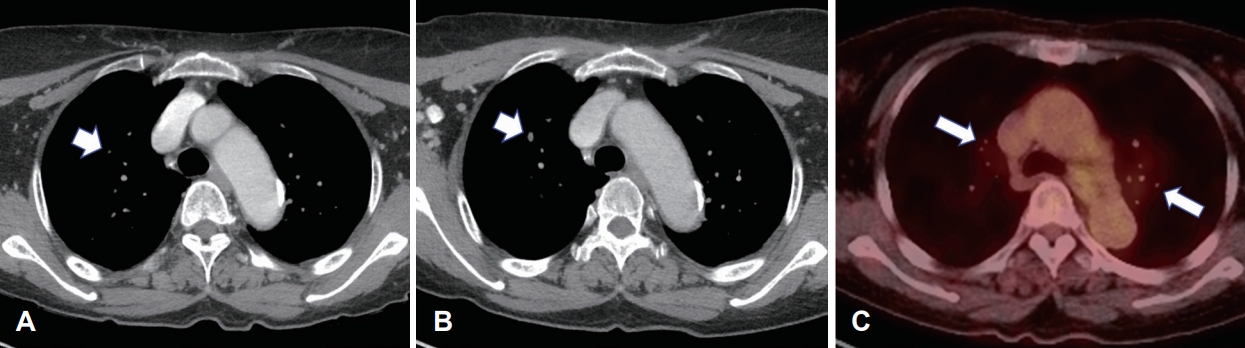
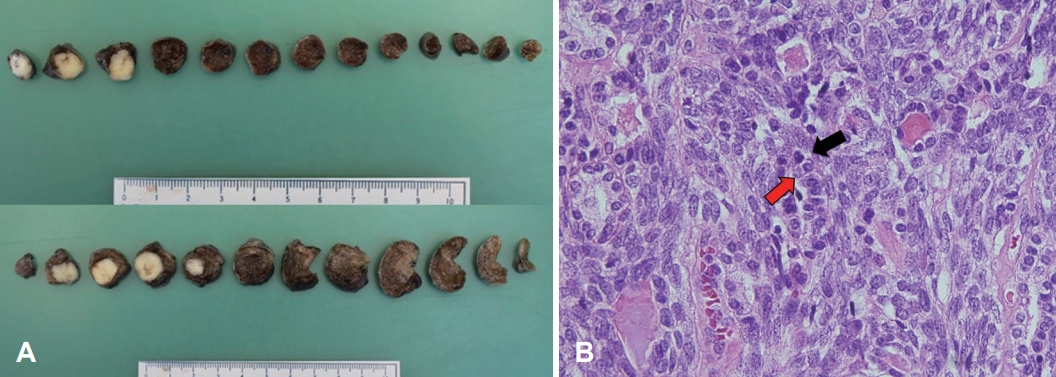
Neck CT at 3 years and 10 months post-PORT showed a 5-mm right upper lung nodule. PET-CT revealed multiple metastatic lung nodules bilaterally. On reviewing the previous contrast-enhanced neck CT, we noted a 0.7-mm right upper lung nodule on neck CT 2 years after PORT, with gradual growth on subsequent neck CTs (Fig. 3). For tissue confirmation, video-assisted thoracoscopic surgery with wedge resection of the right anterior superior and inferior anterior lobes was performed on May 26, 2022. Macroscopically, we noted two solid white lung nodules, size of 1.5×1.2×1.0-cm and 1.5×0.9×0.7-cm with clean-cut surfaces. Microscopically, a biphasic pattern of epithelial cells and myoepithelial cells, resembling the original mass, leads to a diagnosis of metastatic EMCa. To evaluate the potential for targeted therapy, immunohistochemical analysis including androgen receptor and C-erbB2 (HER2) tests were done but both results were negative (Fig. 4).
Finally, a multidisciplinary team of oncologists and pulmonologists reviewed the case and agreed to use adjuvant chemotherapy. From July 6 to September 14, 2022, platinumbased chemotherapy was provided, with three cycles of cisplatin (50 mg/m2, day 1), docetaxel (50 mg/m2, day 1), and 5-fluorouracil (800 mg/m2/day, days 1-4), every 3 weeks. From October 17, one additional cycle using the same regimen was provided. After four chemotherapy cycles, minor regression of metastatic lung nodules was observed on CT. Nevertheless, the disease was considered a “stable disease” according to the Response Evaluation Criteria in Solid Tumors (RECIST). A follow-up chest CT at 3 months after chemotherapy cessation revealed no progression in the lung lesion.
Discussion
The most common EMCa site is the parotid gland (83.7%), followed by the submandibular glands (13.0%) and minor salivary glands (2.4%) [1,2,5]. EMCa has a female-to-male ratio of 1.34:1 and an average age at diagnosis of approximately 63.8 years [5]. Distant metastasis is rare at 2.6% of all cases [6]. The prognosis is generally good [5]. However, distant metastasis lowers the overall survival; the 5-year survival rates decreased from approximately 80% for patients without metastasis to 20% for those with distant metastasis [6]. Since the first report of EMCa in 1972 [8], only 26 cases of metastatic EMCa have been reported, while none have been reported in Korea (Supplementary Table 1) [7]. Among 26 cases, the most common metastasis sites were the lung (17 cases), followed by the liver (3 cases), bone (2 cases), and other sites such as the brain, kidney, skin, and soft tissue (1 case each); moreover, lung metastases co-occurred with brain (2 cases) and bone (2 cases) metastasis and a liver metastasis with brain metastasis. All metastases occurred 1-28 years from the initial diagnosis [9,10]. As hemodynamic metastasis may occur later than the usual 5-10-year follow-up period, long-term observation is vital [11]. One patient had kidney metastasis after six local recurrences over 28 years [9].
Despite the benign-looking histopathologic features of EMCa [11], distant metastasis leads to a poor prognosis [6]. Several histologic features of primary tumors are related to aggressive behavior and a high metastatic rate. Tumors with a solid growth pattern, nuclear atypia, DNA aneuploidy, and high proliferative activity have more aggressive progression and higher local recurrence frequency than tumors without a solid growth pattern [12]. Seethala, et al. [4] reported that three patients died from EMCa with positive margins, angiolymphatic invasion, and primary site necrosis. They all had local or remote metastasis, and two had myoepithelial anaplasia. Therefore, margin status, angiolymphatic invasion, myoepithelial anaplasia, and necrosis are significant predictors of disease-free survival. Our patient had lung metastasis with myoepithelial anaplasia and nuclear atypia. However, no necrosis, angiolymphatic invasion, or positive surgical margins were observed, unlike in other reported cases of metastatic EMCa.
In our case, despite the use of PORT after surgical resection, distant metastasis was found after treatment. A retrospective case review showed pitfalls in diagnosing distant metastasis. Distant metastasis of salivary gland EMCa is rare, therefore regular chest CT appeared unnecessary and was not checked. As a result, the late examination of chest CT delayed the detection of metastasis. For the following reasons, we considered that there was no lymph node metastasis: lymph node metastasis is rare in 3%-4% of all patients, the preoperative neck CT scan showed no lymph node enlargement, and there was no metastasis in the resected lymph node. However, we believe that there was occult lymph node metastasis at the beginning which could have extended to the lung. Although the primary metastatic lesion may be treated with radiotherapy, the metastatic lung lesion is thought to have progressed into multiple metastatic nodules.
Treatment guidelines for metastatic cancer are yet to be established, but chemotherapy can be considered for metastatic lesions. One EMCa case with lung metastasis was treated with three cycles of cisplatin (100 mg/m2, day 1) and 5-fluorouracil (1000 mg/m2/day, day 1-4), every 3 weeks; subsequently, the metastatic lung nodules were stable according to the RECIST [13]. In another case of EMCa with lung metastasis, treated with five cycles of docetaxel (60 mg/m2), cisplatin (60 mg/m2), and fluorouracil (600 mg/m2), complete remission was achieved [14]. Targeted therapy is a potential treatment for metastatic EMCa based on ex vivo findings. In a case of lung metastasis that progressed after chemotherapy, treatment with mammalian target of rapamycin (mTOR) inhibitors resulted in “stable disease” for 11 months [15].
Although distant metastasis is rare in salivary gland EMCa, close post-treatment monitoring, which involves regular follow-up neck and chest CT scans, is important for the early detection of distant metastasis. There are limited reports on metastatic EMCa. Every case report is crucial, as the accumulation thereof will allow physicians to evaluate treatment efficacy and prognosis. Thus, further studies with long-term follow-up after treatment are necessary for establishing the treatment guidelines for metastatic EMCa.