 |
 |
AbstractBackground and Objectives Nasal angioleiomyoma, being a very rare tumor, makes up less than 1% of all angioleiomyomas and is rarely diagnosed preoperatively due to nonspecific and inconclusive radiological and clinical findings. Proper diagnosis and appropriate surgical removal are thus necessary to prevent recurrence. This study focuses on the role of endoscopic transnasal surgery as a minimally invasive approach for the effective treatment of the tumor. We investigated 10 cases to evaluate their diagnosis and surgical outcomes.
Subjects and Method In this study, we retrospectively analyzed 10 patients diagnosed with nasal angioleiomyoma in our hospital from May 2003 to June 2022. We reviewed their data regarding clinical characteristics, diagnosis, treatment, follow-up duration, and recurrence.
Results The diagnosis was more common in females (female:male ratio, 2.3:1) and the mean age at diagnosis was 52.8 years. The most common location was the inferior turbinate, and patients commonly presented with nasal obstruction and epistaxis. The mean tumor size was 1.5 cm. A well-defined, heterogeneously enhancing mass in the nasal cavity was revealed from the CT of the paranasal sinuses. The venous type was most common histologically. All patients underwent transnasal endoscopic excision, and there were no recurrences.
Conclusion Nasal angioleiomyoma typically manifests as a small, painless mass, and we found that transnasal endoscopic excision is a minimally invasive and useful treatment technique with excellent outcomes. For the accurate diagnosis of nasal angioleiomyoma, which is challenging to clinically diagnose, it would be necessary to consider a differential diagnosis and conduct detailed histopathological examination.
IntroductionAngioleiomyomas or vascular leiomyomas are rare tumors that usually arise in vascular smooth muscle of thick-walled vessels [1]. Angioleiomyomas originating in the head and neck regions account for approximately 10% of all angioleiomyomas, and angioleiomyomas are extremely rare in the nasal cavity [2].
The first case of nasal angioleiomyoma was reported by Maesaka, et al. in 1966, and several case reports have since been published [3-5]. Patients commonly present with nasal obstruction or epistaxis. The true etiology is largely unknown. They are rarely diagnosed preoperatively as the clinical and radiological examinations are often nonspecific and inconclusive. Clinicians may have trouble differentiating angioleiomyomas from other benign tumors and establishing further treatment strategies after surgery in the nasal cavity.
Previous studies have documented surgical excision as the recommended treatment [6]. With the introduction of nasal endoscopes, nasal angioleiomyomas can be completely removed through the transnasal endoscopic approach, whereas a few cases may require external approaches such as craniofacial resection. In Korea, while external surgical approaches were previously common for treating nasal angioleiomyomas, endoscopic transnasal excision has become the preferred method since 2008 due to its minimally invasive technique. We investigated 10 cases of nasal angioleiomyoma to evaluate their diagnosis and surgical outcomes.
Subjects and MethodsThis was a retrospective study. Medical records of patients diagnosed with nasal angioleiomyomas at Korea University Guro Hospital between May 2003 and June 2022 were reviewed.
Total of the ten cases were identified from a single tertiary medical center. Patient demographics and clinical characteristics including sex, age, presenting symptoms, location of the tumor, preoperative evaluations, treatment methods and recurrence were evaluated.
The pathology slides were reviewed and confirmed by independent two pathologist using the MorimotoŌĆÖs subtype classification [7]. This study was approved by the Institutional Review Board of Korea University Guro Hospital (IRB No. 2024GR0262).
ResultsClinical characteristicsClinicopathological characteristics of the ten patients are summarized in Table 1. Seven patients were female, three patients were male, and the mean age was 52.8 years (range 25-68 years). The site was in the inferior turbinate (50.0%), nasal septum (20%), nasal floor (20%), and vestibule (10%) in descending order. Common symptoms were nasal obstruction (90.0%) and epistaxis (40.0%). Tumor size ranged from 0.6 to 3.7 cm (mean 1.5 cm)
Diagnosis, treatment, and prognosisUpon physical examination, nasal endoscopy revealed a smooth, round, and vascular-appearing mass with a relatively regular margin (Fig. 1).
All patients underwent preoperative evaluation of the paranasal sinuses using CT. A well-defined, heterogeneously enhanced, vascular lesion was revealed in contrast-enhanced CT. In case 5, the patient presented with epistaxis from the right nasal cavity. A sacciform hemorrhagic mass occupying posterior ethmoid sinus was noted from the nasal endoscopy. Additional MRI scan of paranasal sinuses was conducted, and MRI scan also revealed well-enhanced, hypervascular tumor with a high signal intensity on enhanced T1-weighted image and a mild high signal intensity on the T2-weight image, originating from the nasal septum (Fig. 2).
Preoperative biopsy was not indicated due to the risk of bleeding, and the patient underwent angiography with embolization before surgery because of the large-sized tumor and the expected risk of intraoperative bleeding. Angiography identified the vascular tumor originating from the lateral nasal branch of the right facial artery (Fig. 3). None of the patients was preoperatively diagnosed with angioleiomyoma, and transnasal endoscopic excision was performed in all patients. Histopathological examination confirmed the diagnosis of angioleiomyoma, and no significant intraoperative complications such as bleeding, were encountered in any case.
Follow-up period after surgical treatment ranged from 6 to 60 months, and no recurrence was observed in any of the patients.
Histopathologic findingsIn hematoxylin and eosin stain, tumors were well-circumscribed with thick muscular walls surrounding abundant vascular spaces. Nuclear atypia, necrosis, or mitosis was not observed in the tumor cells (Fig. 4). According to MorimotoŌĆÖs classification, 6 cases (60%) were venous type, 3 cases (30%) were solid type, and 1 case (10%) was cavernous type (Table 1).
Immunohistochemical staining showed positive for desmin, ╬▒-smooth muscle actin (SMA) indicating smooth muscle proliferation and for CD34 highlighting vascular endothelial cells. Tumors were negative for cytokeratin and S-100 protein. These histological findings are consistent with the diagnosis of angioleiomyoma in the nasal cavity.
DiscussionNasal angioleiomyomas are benign soft tissue neoplasms that arise from smooth muscle in the nose. These tumors are even more rare, probably due to the paucity of smooth muscle in the nasal cavity, and they are known to originate from the inferior turbinate, nasal septum, paranasal sinus, or nasal vestibule in descending order [4]. Until now, 11 cases of sinonasal angioleiomyoma have been reported in the Korean literature (Table 2) [8-16].
Nasal angioleiomyomas are usually located in the nasal septum, nasal turbinates, vestibule, and nasal floor [17]. In our study, we could only identify ten cases of nasal angioleiomyoma during our 20-year experience, and the most common location was inferior turbinate.
The causes of nasal angioleiomyoma are unknown, while trauma, virus, steroid therapy and hormonal imbalance have been implicated in the past [5,18]. Angioleiomyomas are characterized by smooth muscle proliferation with varying levels of vascular components [19].
Angioleiomyomas occur more frequently in women showing a peak incidence in the fourth to sixth decades of life [7]. In our study, 70% of the enrolled patients were women and 60.0% of our patients were in the fourth to sixth decades of life, while the remaining patients were in the seventh decade.
The common symptoms of nasal angioleiomyoma are nasal obstruction, epistaxis, facial pain and headaches [17]. There is no characteristic radiological finding for angioleiomyoma. However, CT or MRI is needed to identify extent of tumour and surgical planning. The only way to determine the diagnosis is surgical excision with histologic examination.
Histopathology of angioleiomyoma is characterized by a well-circumscribed tumor covered by sinonasal respiratory type mucosa, composed of well-differentiated smooth-muscle cells having elongated vesicular blunt-ended nuclei with inconspicuous bundles and whorls encasing numerous vascular channels. There is no nuclear atypia or mitosis.
Angioleiomyoma is classified into three histopathologic types according to vascular component of smooth muscle; solid or capillary, cavernous, and venous type [7]. In head and neck region, venous type occurs more frequently and the solid type is smaller than the other types. However, the histologic subtypes have no difference in their clinical prognosis [7,20]. In the meta analysis conducted in 2022, the solid type was the most frequent subtype in angioleiomyomas of the sinonasal tract [17]. However in our study, the frequency of the subtype is venous type in 6 (60%), solid type in 3 (30%) and cavernous type in 1 (10%).
In immunohistochemical staining, angioleiomyomas classically show positivity for smooth muscle markers such as ╬▒-SMA, desmin, vimentin, h-caldesmon and calponin, while showing negativity for melanocytic markers (e.g., HMB45), epithelial markers (e.g., cytokeratin), and neural markers (e.g., S-100) [21-23]. In our study, the tumors are immunopositive for ╬▒-SMA, desmin, and CD34. Nasal angioleiomyomas need to be differentiated from other benign or malignant tumors such as angiofibroma, hemangioma, leiomyosarcoma, angiomyolipoma, and myopericytoma, since it influences treatment strategies, prognosis, and long-term follow-up [24,25].
Surgical excision is the best treatment of choice for angioleiomyoma, and endoscopic or open approaches can be used for surgical treatment of angioleiomyoma in the nasal cavity. The surgical methods depend on the size, location, and extent of the tumor. Preoperative embolization may be considered for large tumors with abundant vascular supply [17]. Other surgical treatment, such as radiofrequency or KTP lasers, can also be utilized [26]. In the nasal cavity, a complete transnasal excision can be achieved in most cases. However, for large or extended tumors, Caldwell-Luc, medial maxillectomy, or craniofacial resection could also be considered [6]. In Korea, external approaches such as open rhinoplasty, lateral alotomy, and sublabial approach were used for nasal angioleiomyomas in the past, but endoscopic transnasal excision has been the mainstream since 2008. Endoscopic transnasal surgery is a simple, rapid, safe, aesthetic, and efficient procedure for the treatment of benign nasal tumors. We performed endoscopic transnasal excision with minimal bleeding and the tumor was completely excised. The prognosis of nasal angioleiomyoma is favorable, since no malignant transformation has been reported to date. Recurrence is extremely rare if complete resection is achieved [17]. In our cases, there was no recurrence or malignant transformation as well up to 5 years.
In conclusion, angioleiomyoma is difficult to clinically diagnose, but it should be considered in the differential diagnosis of nasal lesions. The diagnosis of this tumor requires histopathological evaluation. Complete surgical excision by transnasal endoscopy is recommended for treatment, and the prognosis is favorable. Clinicians need to be aware of clinical course and surgical treatment of an extremely rare angioleiomyoma in the nasal cavity.
Fig.┬Ā1.Nasal endoscopic finding of angioleiomyoma showing round-shaped mass with a relatively regular margin at the head of the right inferior turbinate in patient 4. 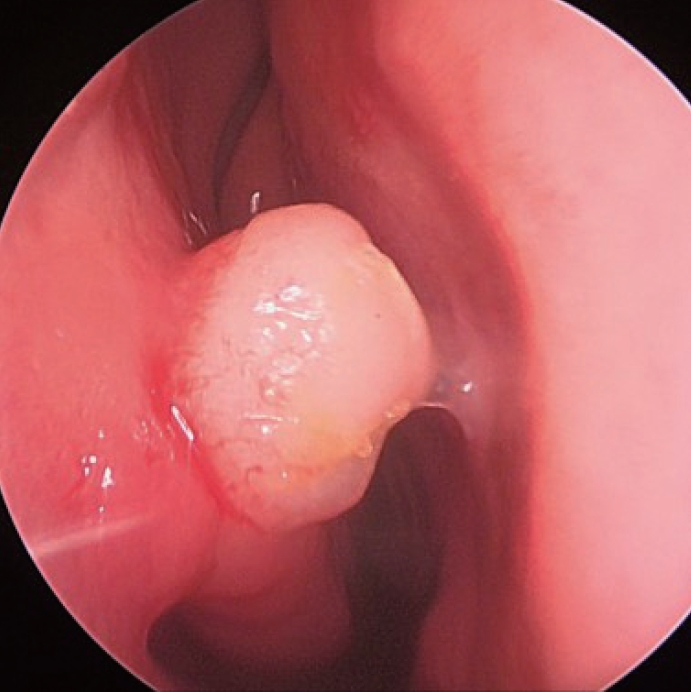
Fig.┬Ā2.Preoperative paranasal sinus CT scan (A and B) and MRI scan (C and D) in patient 5. In axial (A) and coronal (B) views, a heterogeneously enhancing mass was identified in the right ethmoid sinus. This tumor demonstrated a high signal intensity on enhanced T1-weighted image (C), and a mild high signal intensity on the T2-weighted image (D). The asterisk indicates the nasal tumor. 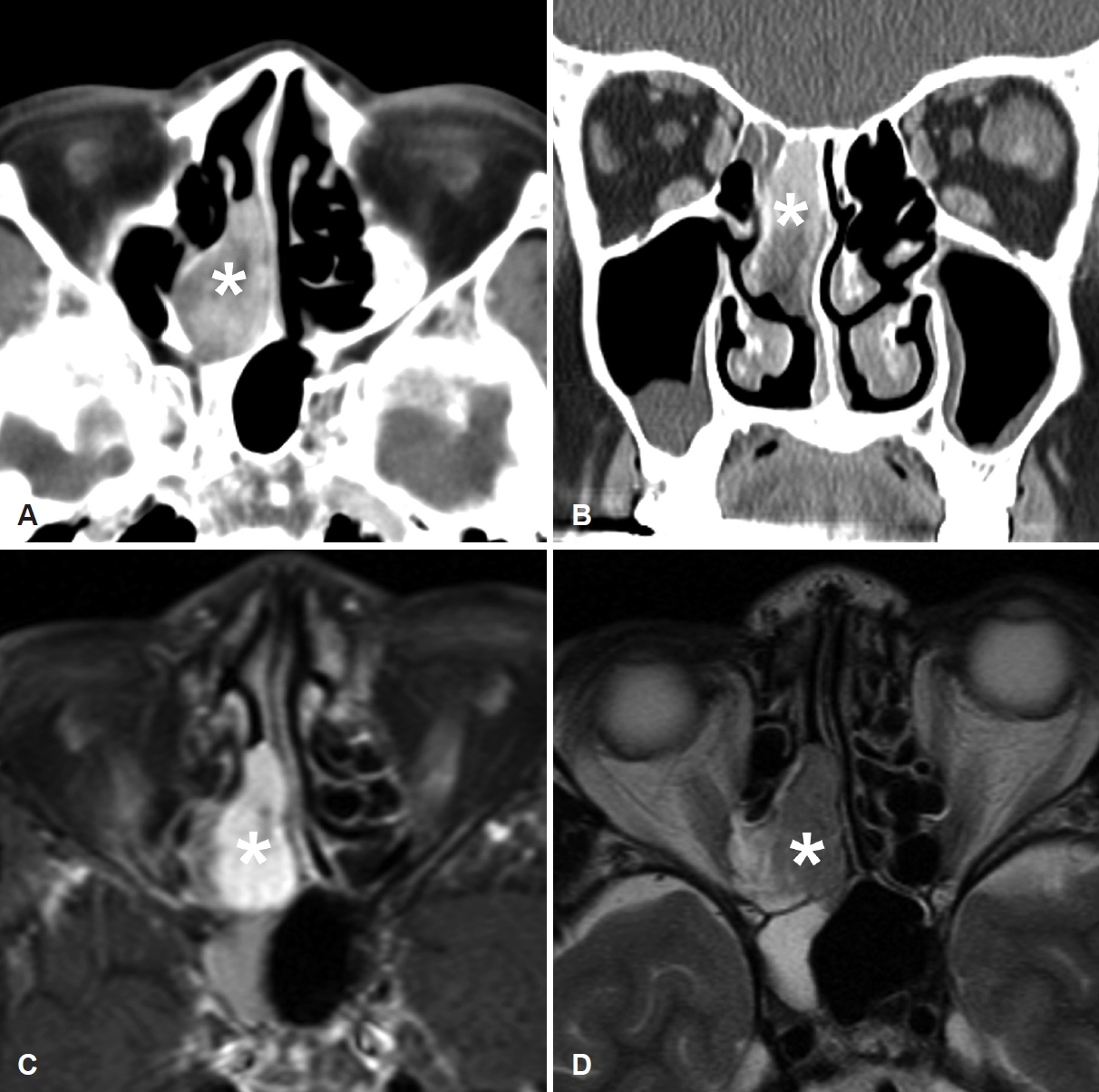
Fig.┬Ā3.Preoperative angiography in patient 5. Blood supply from the lateral nasal branch of the right facial artery was noted (A, black arrow). After embolization, faint staining was still noted, but the blood flow was sluggish (B, white arrow). 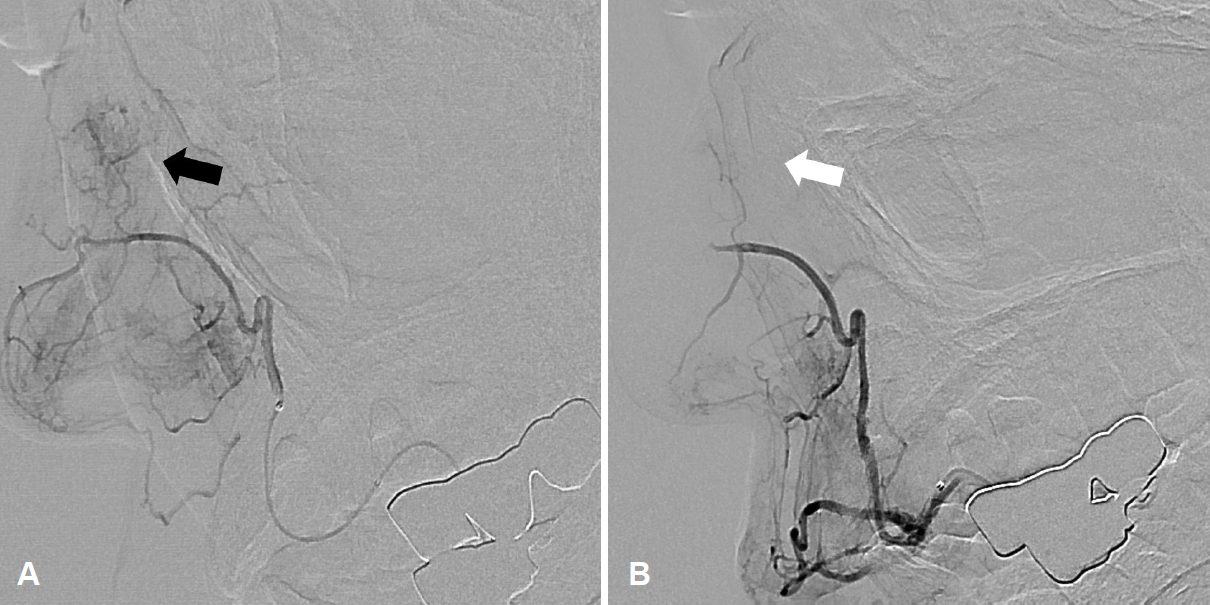
Fig.┬Ā4.Histopathology of angioleiomyoma showing the proliferation of bland spindle cells with eosinophilic cytoplasm and round vessels (A, hematoxylin and eosin). Immunohistochemistry staining showing positivity for desmin (B) and ╔æ-smooth muscle actin (C), and CD34 (D); original magnification ├Ś100. 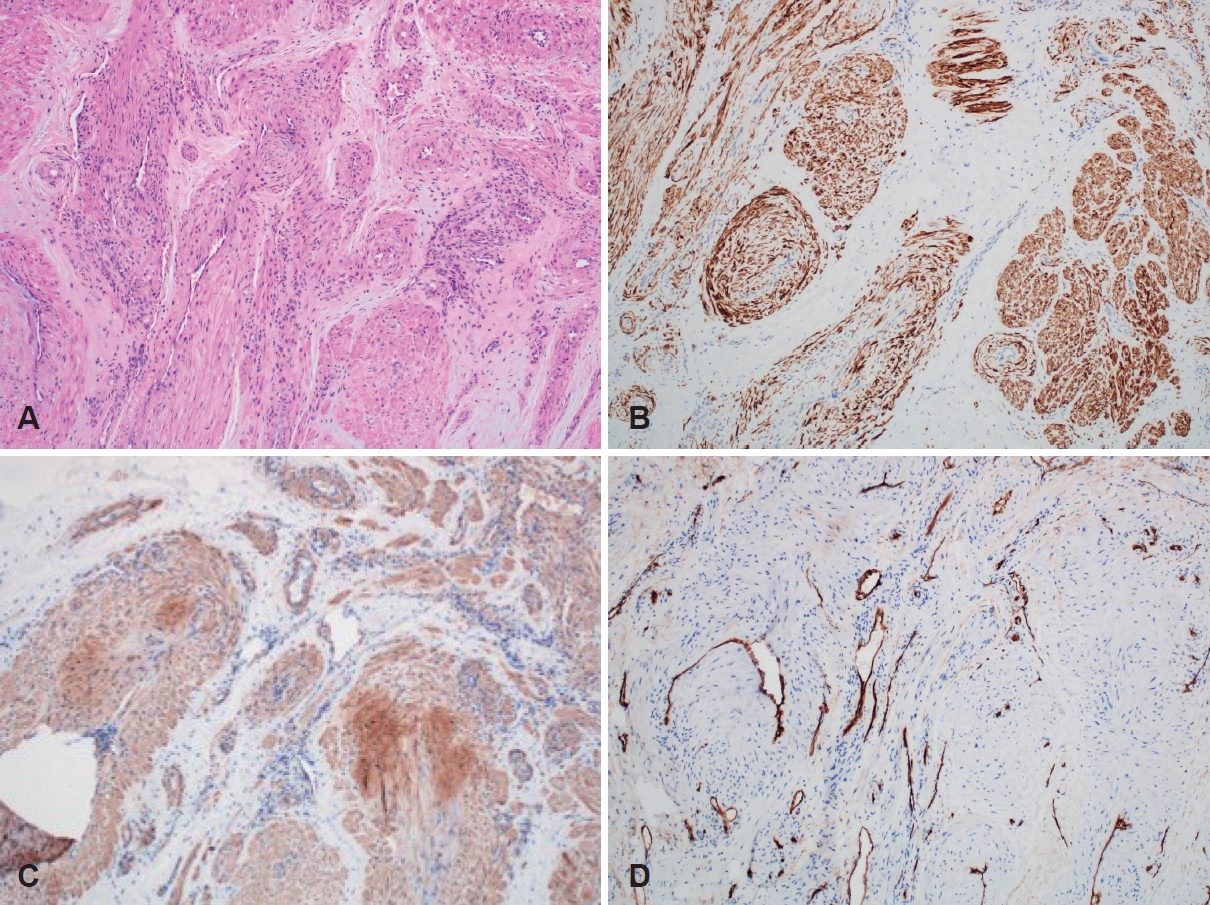
Table┬Ā1.Clinical characteristics of 10 patients with nasal angioleiomyoma Table┬Ā2.Summary of sinonasal angioleiomyoma in Korean literature
REFERENCES1. Agaimy A, Michal M, Thompson LD, Michal M. Angioleiomyoma of the sinonasal tract: analysis of 16 cases and review of the literature. Head Neck Pathol 2015;9(4):463-73.
2. Hachisuga T, Hashimoto H, Enjoji M. Angioleiomyoma. A clinicopathologic reappraisal of 562 cases. Cancer 1984;54(1):126-30.
3. DŌĆÖAguanno V, Ralli M, De Vincentiis L, Remotti D, Minni A, Greco A, et al. Sinonasal angioleiomyoma with adipocyte differentiation: clinicopathologic study of 2 cases and review of the literature. Ear Nose Throat J 2021;100(5):NP222-4.
4. Arora R, Mahindru S, Kathuria K. Sinonasal angioleiomyoma: a rare entity. Biomed Hub 2020;5(2):1-6.
5. Mathieu T, Verbruggen A, Goovaerts G, Declau F. Vascular leiomyoma of the nasal cavity: case report and literature review. Eur Arch Otorhinolaryngol 2017;274(1):579-83.
6. Ho CH, Lin HC, Chou CC, Huang HY. Sinonasal angioleiomyoma. Ear Nose Throat J 2020;99(10):NP109-10.
7. Morimoto N. Angiomyoma (vascular leiomyoma): a clinicopathologic study. Med J Kagoshima Univ 1973;24:663-87.
8. Lee KH, Cho JS, Lee IY, Chang MK. [A case of angioleiomyoma of the nasal dorsum]. Korean J Otorhinolaryngol-Head Neck Surg 2003;46(1):85-7, Korean.
9. Kim SJ, Hong SH, Roh MS. Angioleiomyoma of the nasal cavity. Korean J Pathol 2004;38(3):181-3.
10. Park CS, Cho JH, Kim SW, Park YJ. [Two cases of angioleiomyoma arising from the lateral nasal wall]. J Rhinol 2005;12(1):58-61, Korean.
11. Hwang SH, Chung J, Chung DH, Kim JM. [A case of angioleiomyoma of nasal vestibule]. J Rhinol 2008;15(2):160-3, Korean.
12. Choi JH, Kim JM, Kim YD. Angioleiomyoma of the nasal septum: a case report. J Yeungnam Med Sci 2008;25(2):154-9.
13. Kim YH, Youn SJ, Lee YJ, Kim JS. [A case of angiomyoma of the inferior turbinate]. J Rhinol 2008;15(1):62-4, Korean.
14. Park IH, Hong SM, Choi SW, Shin JM, Lee HM. Two cases of angioleiomyoma in the nasal cavity. J Rhinol 2013;20(2):113-5.
15. Lim SJ, Park YK, Kim HJ. [A case of angioleiomyoma of nasal septum]. Korean J Otorhinolaryngol-Head Neck Surg 2014;57(5):337-9, Korean.
16. Aum JH, Han SW, Kang IG. [A case of angioleiomyoma arising from the right inferior turbinate]. Korean J Otorhinolaryngol-Head Neck Surg 2015;58(5):355-8, Korean.
17. Velletrani G, Maurizi R, De Padova A, Di Girolamo S. Angioleiomyoma of the sinonasal tract: a systematic review of an uncommon clinicopathological entity. Int Arch Otorhinolaryngol 2023;28(2):e350-66.
18. Zhu G, Xiao D, Sun P. Expression of estrogen and progesterone receptors in angioleiomyoma of the nasal cavity of six patients. Oncol Lett 2016;11(4):2359-64.
19. Hisaoka M, Quade B. Angioleiomyoma. In: Fletcher CD, Bridge JA, Hogendoorn PCW, Mertens F, editors. WHO classification of tumor of soft tissue and bone. 4th ed. Lyon: IARC;2013. p.120-1.
20. Perardi F, Abbate G, Iannuzzelli LR, Contini R, De Munari M, Sciuto FG, et al. Angioleiomyoma of the larynx: a case report and literature review. Ear Nose Throat J 2020;99(10):658-63.
21. Liu Y, Li B, Li L, Liu Y, Wang C, Zha L. Angioleiomyomas in the head and neck: a retrospective clinical and immunohistochemical analysis. Oncol Lett 2014;8(1):241-7.
23. Yoon TM, Yang HC, Choi YD, Lee DH, Lee JK, Lim SC. Vascular leiomyoma in the head and neck region: 11 years experience in one institution. Clin Exp Otorhinolaryngol 2013;6(3):171-5.
24. Lee HM, Kim JM, Chu HS, Lee SH. [A case of angiomyoma of the inferior turbinate]. Korean J Otorhinolaryngol-Head Neck Surg 2002;45(12):1193-5, Korean.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

 |
 |