 |
 |
AbstractBackground and ObjectivesThe efficacy of non-invasive neuromodulation techniques for treating tinnitus has been investigated with varying results. The purpose of current study was to evaluate the feasibility of two forms of neuromodulation, the transcutaneous vagus nerve stimulation (tVNS) and the transcranial random noise stimulation (tRNS), as treatments for chronic tinnitus.
Subjects and MethodA total of 24 tinnitus patients were enrolled in the study, with 11 receiving tVNS and 13 receiving tRNS. Four subjective questionnaires and electroencephalogram (EEG) tests were conducted to evaluate the effects of neuromodulation on cortical activity.
ResultsBoth tVNS and tRNS significantly reduced the intensity and distress of tinnitus, as well as Tinnitus Handicap Inventory scores 3 days after treatment. These effects persisted for 1 month with no significant differences between the two treatments. The EEG data revealed a notable decrease in the power of the beta and gamma bands primarily in the auditory cortex on the opposite side of stimulation for both tVNS and tRNS. The decrease in the power of the beta band was more widespread in the tRNS group than in the tVNS group, affecting the entire brain 3 days after treatment. Furthermore, this reduced activity continued for several electrodes up to 1 month after treatment. The correlation analysis indicated a significant relationship between decreased neural activity and reduction in tinnitus symptoms in both treatment groups.
IntroductionNon-invasive neuromodulation techniques have been widely investigated as tinnitus treatments for several decades. Reduced auditory input may increase synchronous firing or spontaneous activity in the auditory neurons [1-4]. This increase in central gain, which compensates for auditory differentiation [5-7], can cause cortical hyperactivity, as shown in animals [4] and human studies [2,8], leading to tonotopic reorganization in the auditory cortex [2]. Therefore, maladaptive neuronal plasticity in the central auditory system is one of the general neurophysiological mechanisms of tinnitus [9,10]. In addition to the auditory cortex, non-auditory networks in the brain that are involved in attention, emotion, and memory are also involved in tinnitus [8,11]. These non-auditory networks may determine the loudness and distress caused by tinnitus, particularly when accompanied by symptoms such as sleep disturbance, anxiety, or depression [12]. In this context, various neuromodulation techniques are being explored as potential treatments for tinnitus, with the aim of reversing the adverse plastic changes that occur in both auditory and non-auditory brain areas. Among the neuromodulation techniques, low-intensity electrical stimulation of specific brain regions has been applied in many clinical research fields, including the improvement of cognitive and learning performance, motor rehabilitation, and pain relief [13-15]. Transcranial direct current stimulation of the temporoparietal cortex [16,17] or dorsolateral prefrontal cortex [18-20] has been investigated for tinnitus control, as has transcranial random noise stimulation (tRNS), which causes less pain and skin irritation by providing balanced current stimulation and can reduce tinnitus loudness in some patients [21-25].
Vagus nerve stimulation (VNS) has been shown to influence the afferent nerve projection to the nucleus of the tractus solitarius in the brainstem and the cholinergic nucleus basalis in the basal forebrain, thereby modulating the release of various neurotransmitters, including norepinephrine, serotonin, and acetylcholine [26-29]. Early animal studies by Zabara [30] demonstrated the effects of VNS on seizure control. Subsequently, VNS received approval from the U.S. Food and Drug Administration for the treatment of intractable epilepsy, followed by its approval in 2005 for chronic or recurrent depression [26]. Furthermore, Kilgard and Merzenich [31] reported that the simultaneous application of sensory stimuli and electrical stimulation of the cholinergic nucleus basalis in rats led to a significant and enduring reorganization of the primary auditory cortex in adult individuals. The promising therapeutic effect of bimodal stimulation of the cervical vagus nerve and auditory signal on tinnitus was demonstrated in an animal study [32] and a human study [33]. Since electrical stimulation of the auricular branch of the vagus nerve offers a non-surgical approach with a reduced risk of cardiovascular side effects, transcutaneous VNS (tVNS) has been utilized as a method for managing tinnitus in several clinical trials [34,35].
The underlying mechanism behind the therapeutic effects of neuromodulation, including tinnitus and other disorders, remains poorly understood [12,36]. However, we hypothesize that optimized neuromodulation may reduce tinnitus-related neuronal hyperactivity, based on previous neuroimaging and neuromodulation studies [12,37,38]. To test this hypothesis, we conducted a clinical study using two forms of neuromodulation: tVNS as an indirect brain stimulation method and tRNS as a direct brain stimulation method. The objective of this study was to assess the feasibility of tVNS and tRNS as subjective tinnitus treatments in patients by using subjective questionnaires and electrophysiological measurements. We also intended to identify the effective brain areas for each type of neuromodulation and to compare the cortical responses between tVNS and tRNS through quantitative electroencephalogram (EEG) analysis.
Subjects and MethodsClinical trial designThis study was a randomized, parallel-group, single-blind trial with a sham-controlled design without a control group. The Randomizer software was utilized from the Phase Locked Software, which is freely accessible to clinical trial researchers. Employing the permuted block randomization algorithm, we were able to equally and randomly allocate the participants into two groups. All the patients were blinded and unaware of which treatment they would receive; only the researcher conducting the treatment was aware of the participantŌĆÖs assigned treatment group. At the first visit, prior to receiving the sham treatment, participants completed all questionnaires and answered questions about their tinnitus symptoms. They also underwent pure tone audiometry and EEG. During the clinical trial, participants received three sessions of sham treatment every other day in the first week before starting the real treatment. After the sham treatment, participants received six sessions of real treatment every other day over a 2-week period, resulting in a total treatment duration of 3 weeks (Fig. 1). Prior to the initiation of real treatment, participants completed questionnaires to evaluate the impact of the sham treatment. To assess the effects of the real treatment, participants returned for follow-up visits and completed questionnaires after 3 days and 1 month upon completion of the real treatment. Consequently, participants attended a total of 11 visits (Fig. 1). Throughout the clinical trial, none of the participants were informed about the sham treatment as the study was designed as a single-blind trial with a sham-controlled design.
The clinical efficacy of the treatment was assessed through questionnaires about tinnitus symptoms and 5 min of resting-state EEG at the following four times: 1) before treatment (EEG and questionnaire); 2) after sham treatment (questionnaire only); 3) immediately after the sixth session of real stimulation (EEG only); 4) 3 days after treatment (EEG and questionnaire); and 5) 1 month after treatment (EEG and questionnaire). The clinical trial was approved by the Institutional Review Board (IRB) of Eulji Medical Center (IRB No. EMCIRB 18-69) and the first participant was enrolled on 12/06/2019, registration number 1. Throughout the whole clinical trial period, the researcher conducted the clinical trial in compliance with the revised Helsinki Declaration of 2000. The study protocol was registered with the Clinical Research Information Service (KCT0007520, https://cris.nih.go.kr/cris/search/detailSearch.do/21987) and was approved by the Korean Food and Drug Administration (No. 957) before initiation. On the first day of the trial, all participants provided written informed consent.
SubjectsIn this study, we included patients who met the following four criteria: 1) tinnitus duration of more than 2 months; 2) over 18 years of age; 3) complaints of tinnitus discomfort; and 4) refractory to pharmacotherapy or tinnitus-retraining therapy. Patients who met at least one of the following criteria were excluded: 1) diagnosed with other otologic diseases, such as otitis media, acoustic tumor, or M├®ni├©reŌĆÖs disease; 2) tinnitus accompanied by sudden hearing loss; 3) history of use of ototoxic drugs; 4) suspected somatic tinnitus with complaints of the temporomandibular joint and cervical pain; 5) objective tinnitus, such as pulsatile tinnitus; 6) history of arrhythmia and asthma and implantation of heart pacemaker or implantable cardioverter-defibrillator; and 7) pregnancy.
The drop-out rate over the entire clinical trial period was 23.6% (4/17) for the tRNS group and 35.3% (6/17) for the tVNS group with no statistically significant difference observed between the two groups (p=0.452). During the sham treatment, two participants in the tRNS group dropped out, and during the real treatment, another participant from the tRNS group dropped out. During the one-month follow-up period after treatment, five participants in the tVNS group and one in the tRNS group dropped out. Data from 24 tinnitus patients were analyzed (tVNS, n=11; tRNS, n=13) (Fig. 2).
The mean age of the patients was 53.9 (standard deviation [SD]=17.5) years, with 14 male and 10 female. The tinnitus duration ranged from a minimum of 2 months to a maximum of 240 months (mean=55.9 months, SD=61.2). The pure-tone averages at frequencies of 500, 1000, 2000, and 3000 Hz in the tVNS group were 22.4 (SD=10.9) dB hearing level (HL) in the right ear and 25.0 (SD=10.4) dB HL in the left ear. In the tRNS group, pure-tone averages were 21.0 (SD=16.9) dB HL in the right ear and 19.9 (SD=15.6) dB HL in the left ear. Thresholds for each frequency are presented in Supplementary Table 1, and there were no significant differences in the pure-tone average between the tRNS and tVNS groups for either ear (p> 0.05). Additionally, there were no significant differences in age (p>0.05), tinnitus duration (p>0.05), or the scores of the four questionnaires administered before treatment (p>0.05) between the groups. The mean THI score before treatment was 44.5 (range: 10 to 94, SD=29.8) in the tVNS group and 41.8 (range: 12 to 88, SD=23.7) in the tRNS group. Detailed means and ranges for each questionnaire can be found in Supplementary Table 2.
tVNSWe targeted the cavum concha of the left auricle with tVNS by using an electrical stimulator (TENS eco 2; Schwa-medico, Ehringshausen, Germany) based on the same settings used in our previous study [35]. A 2-cm-diameter circular electrical pad was bent to attach it to the tragus and cavum concha. To avoid the potential effects of tVNS on the heart, all participants underwent stimulation on the left ear, regardless of their tinnitus side. The electrical stimulator used monophasic pulsed current stimulation with a pulse rate of 20 Hz, intensity of 2 mA, and pulse width of 200 ╬╝s. Participants had their blood pressure, heart rate, and oxygen saturation measured before and after tVNS, which lasted for 20 min. The anodal pad was attached to the auricle and the cathodal pad was attached to the cervical muscle. The electrical stimulator used in this study had two electrode channels, with two electrodes per channel. For the real stimulation of tVNS, the settings were exclusively applied to channel 1, while channel 2 was designated for sham tVNS. During the sham stimulation period, no actual current was delivered through the electrodes placed at the same site as the real stimulation. However, the stimulatorŌĆÖs screen displayed the ongoing operation, giving the impression of active stimulation. During the stimulation, participants listened to classical music mixed with low-intensity nature sounds.
tRNStRNS was applied using a DC stimulator (DC-stimulator Plus, NeuroConn, Germany) that provided both electrical random noise stimulation and sham stimulation. Two 4.8├Ś4.8 cm electrical pads were attached to the temporal area and stimulated with a low frequency of 0.1 to 100 Hz at 2 mA for 20 min. To avoid potential neural hyperactivity in the temporal lobe, the anodal stimulation was performed on the opposite side to the tinnitus side and the cathode on the same side, based on our pilot study. The cathode and anode were attached above the ears to affect Brodmann areas 20, 21, 22, 41, and 42, including the primary and secondary auditory cortex. In the sham condition, only a small current pulse will occur every 550 ms (110 ╬╝A over 15 ms) instead of the stimulation current. The peak current of sham stimulation lasts for 3 ms and the average current over time is not more than 2 ╬╝A, which has no therapeutic effect. Participants listened to classical music mixed with low-intensity nature sounds during stimulation.
QuestionnairesTo evaluate tinnitus symptoms subjectively, four questionnaires were conducted: 1) tinnitus intensity (10-point visual analog scale); 2) tinnitus distress (10-point visual analog scale); 3) THI (a maximum score of 100); and 4) BDI (a maximum score of 63). Tinnitus intensity and distress questionnaires were conducted four times (before treatment, after sham treatment, 3 days after treatment, and 1 month after treatment), whereas THI and BDI were conducted three times, excluding after sham treatment.
EEGTo evaluate the changes in cortical activity due to neuromodulation, we conducted EEG tests on participants. The participants were asked to abstain from smoking and drinking alcohol for over 12 h before the test. On the day of the EEG, participants were seated in a quiet and electrically shielded room maintained at a temperature of 22-26┬░C. The participants were instructed to keep their eyes closed for 5 min of the EEG test, during which they remained in a resting state. We collected EEG data from 32 channels using the actiCHamp Brain Products recording system (Brain Products GmbH, Munich, Germany). The signal was sampled at a rate of 500 Hz. Two electrodes served as reference electrodes and were attached to both sides of the mastoid bone, and electrooculographic signals were recorded simultaneously using a surface electrode placed below the left eye. To eliminate artifacts, we applied a band-pass filter (0.1-60 Hz), and ocular noise was removed using Independent Component Analysis implemented in BrainVision Analyzer 2. The spectra were calculated using the full range of frequencies from the EEG data collected over a continuous recording period of 5 minutes. No epochs were rejected from the recorded EEG data, and the entire 5-minute recording was used for the spectral analysis. In the statistical analysis, we excluded the Fp1 and Fp2 electrodes owing to potential contamination from eye movement artifacts. Additionally, FT10 was excluded because the neuronal activity before treatment differed between both groups. Consequently, the statistical analysis was conducted on a total of 26 electrodes, specifically: Fz, F3, F7, FT9, FC5, FC1, C3, T7, CP5, CP1, Pz, P3, P7, O1, Oz, O2, P4, P8, CP6, CP2, C4, T8, FC6, FC2, F4, and F8. All electrode impedances were kept below 15 k╬®. The EEG data preprocessing and scalp map results were derived using BrainVision Analyzer ver. 2. The spectral analysis was performed using the fast Fourier transform embedded in BrainVision Analyzer. The frequency resolution was 1 Hz with a Hanning window, and the passband was 0.5-50 Hz. The fast Fourier transform output was in the form of power (╬╝V2) and power density (╬╝V2/Hz). The analysis was conducted in the following eight frequency bands: delta (2.0-3.5 Hz), theta (4.0-7.5 Hz), alpha-1 (8-10 Hz), alpha-2 (10-12 Hz), beta-1 (13-18 Hz), beta-2 (18.5-21.0 Hz), beta-3 (21.5-30.0 Hz), and gamma (30.5-44.0 Hz).
Statistical analysisA mixed two-way repeated-measures ANOVA was conducted to assess the effect of time on tinnitus symptom changes and to compare the effects of tVNS and tRNS. The post hoc analysis for the changes in tinnitus symptoms at different time points was conducted using the Bonferroni correction. The EEG neural activity before and after treatment was compared using the Mann-Whitney U test with the BonferroniŌĆÖs correction (╬▒=0.05/3=0.017), and the correlation between changes in tinnitus symptoms and neural activity was evaluated using non-parametric Spearman analysis. The statistical analyses were performed using PASW 18 (IBM Corp., Armonk, NY, USA).
ResultsTinnitus questionnaire resultsRepeated measures analysis of variance (ANOVA, four time points├Śtwo groups) for tinnitus intensity, tinnitus distress, and Tinnitus Handicap Inventory (THI) score showed a significant main effect of time on tinnitus intensity [F (1.885, 33.38)=11.438, p<0.001], tinnitus distress [F (2.225, 33.377)=9.701, p<0.001], and THI score [F (2, 26)=8.895, p=0.001]. However, there was no significant difference between the tVNS and tRNS groups (all p>0.05), nor any significant interactions between time and group (all p>0.05). There was no significant main effect for time on BeckŌĆÖs Depression Inventory (BDI) scores [F (2, 26)=2.364, p=0.114] and no significant difference between the tVNS and tRNS groups [F (1, 13)=0.006, p=0.942] (Table 1). The mean tinnitus intensities were 6.24 (SD=2.0) before treatment, 6.15 (SD=1.8) just after sham treatment, 5.32 (SD=1.8) at 3 days after treatment, and 4.88 (SD=1.6) at 1 month after treatment. The mean tinnitus distress scores were 6.03 (SD=2.2) before treatment, 5.50 (SD=2.5) just after sham treatment, 4.68 (SD=2.0) at 3 days after treatment, and 4.47 (SD=1.5) at 1 month after treatment. The Bonferroni post hoc test revealed significant decreases in tinnitus intensity and distress from before treatment to 3 days after treatment and 1 month after treatment (p=0.021 and p=0.006 for tinnitus intensity; p=0.012 and p=0.004 for tinnitus distress) (Table 2 and Fig. 3). Compared with the sham treatment, there was a significant decrease in tinnitus intensity from just after sham treatment to 1 month after treatment (p=0.009) and in tinnitus distress from just after sham treatment to 3 days after treatment (p=0.047). The THI scores were 46.2 (SD=25.2) before treatment, 34.5 (SD=18.7) at 3 days after treatment, and 34.1 (SD=16.5) at 1 month after treatment. A significant decrease in THI score was observed from before treatment to 3 days after treatment and 1 month after treatment (p=0.011 and p=0.015, respectively) (Table 2 and Fig. 3).
The participants in this study were defined as responders if they met the criteria of experiencing a decrease of more than 1 point on the 10-point scale for both tinnitus intensity and distress, as well as a decrease of 10 points on the THI score. Three days after treatment, the total number of respondents was 15/24 (62.5%, tVNS: 5/11, tRNS: 10/13) for tinnitus intensity, 16/24 (66.7%, tVNS: 5/11, tRNS: 11/13) for tinnitus distress, and 13/24 (54.2%, tVNS: 6/11, tRNS: 7/13) for THI score. One month after treatment, the total number of respondents was 13/24 (54.2% tVNS: 5/11, tRNS: 8/13) for tinnitus intensity, 13/24 (54.2% tVNS: 5/11, tRNS: 8/13) for tinnitus distress, and 8/24 (33.3% tVNS: 3/11, tRNS: 4/13) for THI score.
EEG results
Fig. 4 shows the comparisons for tVNS and tRNS neuromodulation before and after treatment on scalp maps. The change in the neural activity was calculated by subtracting the data before treatment from the data at each time point after treatment. The cortical activity of participants who received tRNS was lower than those who received tVNS; thus, the color scale of the scalp maps was adjusted by ┬▒170 ╬╝V for tVNS and ┬▒42.5 ╬╝V for tRNS in Fig. 4A. Both tVNS and tRNS demonstrated a decrease in neural activity across various brain areas compared to pre-treatment levels (Bonferroni corrected p value of <0.017), with the maximum effect observed at 3 days after treatment. Both the tVNS and tRNS groups showed significantly decreased beta activity in the temporal cortices after treatment, suggesting that the stimulation affected the temporal cortices in both groups (Bonferroni corrected p value of <0.017). In the tVNS group, T8, located on the opposite side of the stimulation to the temporal cortex (blue circle), showed a significantly decreased power density of beta activity 3 days after treatment. However, this effect was not sustained until 1 month after treatment (Fig. 4A). Fig. 4B also shows a decrease in the power of beta activity at Fz, F7, O2, CP2, T8, FC6, FC2, and CP6 in the tVNS group 3 days after treatment (Supplementary Tables 3-5). In contrast, the tRNS group exhibited a decrease in beta band power across several electrodes immediately after treatment, with a broader effect observed throughout the entire brain 3 days after treatment. This reduced activity continued for several electrodes up to 1 month after treatment (Fig. 4A, C, and Supplementary Tables 6-8). Although decreased beta activity was observed at both bilateral temporal cortices (T7 and T8, blue circle) 3 days after treatment, the decrease in neural activity was only maintained at the right temporal cortex until 1 month after treatment (Fig. 4A and C). Additionally, a significant decrease in beta activity (p<0.017) was observed in CP6 and FC6 electrodes, near the right temporal lobe, at 3 days after treatment, and these decreases persisted up to 1 month after treatment (Fig. 4A and C).
Correlation between changes in tinnitus symptoms and neural activityThe correlation analysis indicated a significant relationship between the decrease in neural activity and the reduction in tinnitus symptoms for tVNS and tRNS. tVNS had a strong positive correlation between changes in neural activity in the left frontal, central, and parietal cortex and THI scores 3 days after treatment. Additionally, tVNS showed a weak positive correlation between changes in neural activity in multiple electrodes and tinnitus distress 3 days after treatment (Fig. 5A). tRNS displayed a significant positive correlation between changes in neural activity, particularly in the beta and gamma bands, and both tinnitus distress and tinnitus intensity 3 days after treatment (Fig. 5B). However, tRNS also showed significant negative correlations between changes in neural activity in the delta and theta bands and tinnitus intensity 3 days after treatment. These findings suggest that tVNS and tRNS may have different mechanisms of action in modulating neural activity and improving tinnitus-related outcomes.
DiscussionThe results of our study add to the growing body of evidence on the subjective therapeutic effects of tVNS and tRNS in tinnitus patients. Previous studies have reported reductions in tinnitus loudness, tinnitus distress, and THI scores from 10% to 68% in tinnitus patients treated with tVNS [39]. Vanneste, et al. [40] also reported a significant reduction in THI scores in tinnitus patients who received long-term tVNS stimulation with a frequency of 30 Hz [40]. The use of low-frequency tRNS stimulation (0.1-100 Hz) in our study is consistent with previous research, which has shown that low-frequency tRNS stimulation is more effective in reducing tinnitus symptoms than high-frequency stimulation [21,23-25]. Increased tinnitus loudness after high-frequency tRNS stimulation has been reported [41]. Although the in vivo and cellular mechanism underlying transcranial stimulation is not yet fully understood, low-frequency electrical stimulation can induce synaptic depression [42-44], whereas high-frequency stimulation can lead to a late phase of long-term potentiation, which is associated with memory and neuronal connectivity [43]. Furthermore, high frequencies often result in increased activity amplitudes, peak numbers, and neural connectivity [42,43,45]. Based on our findings, both tVNS and tRNS treatments cause significant reductions in tinnitus intensity, tinnitus distress, and THI scores 3 days after treatment, as well as sustaining these reductions 1 month after treatment, suggesting that these treatments provide rapid and sustained relief for tinnitus patients. Importantly, the effectiveness of tVNS and tRNS treatments in reducing tinnitus intensity and distress is further supported by the absence of similar changes observed in the sham treatment. The lack of changes in BDI scores after treatment might be attributed to the relatively low baseline BDI scores in both treatment groups (tVNS: 14.50┬▒17.72; tRNS: 13.11┬▒7.98).
Our analysis of EEG data revealed that both tVNS and tRNS interventions induced a significant decrease in power in the beta-1, beta-2, beta-3, and gamma bands. This is consistent with previous research exploring the effects of tVNS on tinnitus patients, including magnetoencephalography studies that found that the amplitude of the auditory N1 response decreased [34] and that the reduction in beta and gamma band synchrony was correlated with tinnitus severity [46]. Yakunina, et al. [39] found that tVNS can deactivate brain areas related to tinnitus generation and distress, including limbic areas. A study on tRNS for tinnitus treatment also showed an increase in alpha-1 band power in the auditory and prefrontal cortex, as well as a decrease in delta and beta-2 band power in the prefrontal cortex [24].
The decrease in neural activity was greatest 3 days after treatment, and the reduction in neural activity persisted for up to 1 month, primarily in the temporal cortex. The strongest downregulation effect was observed in the electrodes around the temporal lobe, suggesting that the stimulation protocol mainly affected this region. Even though tVNS was administered on the left side, it caused a substantial decrease in beta-2 and beta-3 activities on the right auditory cortex, indicating that the stimulation affected the opposite hemisphere of the temporal cortex. On the other side, tRNS elicited bilateral stimulation of the temporal lobes and resulted in reduced neural activity in both auditory cortices, with the effect persisting in the right temporal cortex up to 1 month after treatment. Furthermore, significant decreases were observed in the CP6 and FC6 electrodes, predominantly around the right temporal lobe, rather than the left. These decreases were found 3 days after treatment and persisted for up to 1 month thereafter. Among the tRNS recipients, 10 underwent left cathodal stimulation and three underwent right cathodal stimulation based on their tinnitus side. Given that cathodal stimulation produces an inhibitory effect [47,48], left cathodal stimulation appeared to have a more potent effect on the opposite side of the temporal cortex, leading to a longer-lasting effect. Previous studies have reported responses in the opposite hemisphere to brain stimulation, with one-sided stimulation activating or deactivating the opposite side of the brain [49-50]. However, some differences were noted between tVNS and tRNS in terms of timing and the range of the cortex affected. Although both tVNS and tRNS led to a decrease in neural activity at a few electrodes immediately after treatment, tRNS exhibited a more potent and broader range of decline 3 days after treatment compared to tVNS. Specifically, tRNS produced a significant reduction in beta band power not only in the auditory cortex but also in the frontal, parietal, and occipital cortex 3 days after treatment, with the reduction effect lasting for 1 month in more electrodes than tVNS. These findings suggest that tRNS may be more effective in addressing symptoms related to tinnitus, such as emotional or concentration problems, despite the absence of differences between the two neuromodulation stimuli in subjective symptom changes. Several reasons may explain the differences in the change of neural activity between tVNS and tRNS. First, there are differences in the location and size of the electrical pads used for stimulation. tRNS uses two 4.8├Ś4.8 cm pads applied to the temporal area, whereas tVNS uses a 2-cm-diameter circular pad attached to the bend of the tragus in the cavum concha. Second, the differences in the mechanism of action of the stimuli themselves may also play a role. tVNS targets the auricular branches of the vagus nerve, stimulating the noradrenergic locus coeruleus in the brainstem and the cholinergic nucleus basalis in the basal forebrain, leading to cortical reorganization through the release of neurotransmitters, such as acetylcholine [26-29]. In contrast, tRNS modulates neural asynchrony by randomly stimulating the temporal lobe with electrical impulses.
Our EEG data and the correlation between changes in tinnitus symptoms and neural activity commonly suggest that reducing neural activity in high-frequency bands (beta and gamma) can alleviate tinnitus symptoms. Interestingly, increases in neural activity in low-frequency bands (delta and theta) appear to be involved in reducing tinnitus intensity during tRNS. This finding is consistent with the theory that maladaptive plastic changes in the auditory cortex are important in the development of tinnitus [10].
The new findings in this study on neuromodulation for treating subjective tinnitus can be summarized as follows. First, the downward modulation of high-frequency bands may alleviate tinnitus symptoms, whereas low-frequency bands may have the opposite effect on tinnitus control. Second, both tVNS and tRNS mainly affected neuronal activity in the auditory cortex on the opposite side of stimulation. Third, tRNS induced a faster reduction in neuronal activation and showed a wider efficacy over brain areas than tVNS.
There are several limitations in the present study. The sample size was relatively small due to the reluctance of patients to visit the hospital during the COVID-19 pandemic, and this resulted in a dropout rate of 10 patients during treatment and follow-up. Given the heterogeneity of patient symptoms, the small sample size in this study requires careful attention when interpreting the results. In addition, there is a technical limitation for tVNS. In previous studies of tVNS, the stimulus intensity was often tailored to the individualŌĆÖs pain threshold [34,35,39]. However, in this study, the tVNS intensity was fixed at 2 mA to align with the intensity used in tRNS. Because the electrical pad used for tVNS was not designed for ear stimulation its larger size and the curved anatomy of the cavum concha may have led to weaker adhesion to the skin, resulting in a reduction in stimulation power. This could have led to a reduction in stimulation power, introducing potential discrepancies in stimulation intensity between tVNS and tRNS, which might have influenced the obtained results. Additionally, the relatively large electrode pad may have inadvertently stimulated other nerves innervating the outer ear apart from the vagus nerve, potentially contributing to the observed effects.
In conclusion, this clinical study supports both tVNS and tRNS as promising therapeutic options for individuals suffering from tinnitus. The EEG data suggest that tVNS and tRNS may have different mechanisms of action in modulating neural activity and improving tinnitus-related outcomes. The correlation analysis indicates a significant relationship between decreased neural activity and a reduction in tinnitus symptoms using tVNS and tRNS. Further research is needed to optimize the use of these treatments in clinical practice.
Supplementary MaterialThe Supplement is available with this article at https://doi.org/10.3342/kjorl-hns.2024.00409.
Supplementary Table 1. Mean and SD of hearing thresholds
kjorl-hns-2024-00409-Supplementary-Table-1.pdf
Supplementary Table 2. Tinnitus questionnaire results at each time point
kjorl-hns-2024-00409-Supplementary-Table-2.pdf
Supplementary Table 3. Comparison of cortical activity immediately after treatment and before treatment in the tVNS group (correlated with Fig. 4)
kjorl-hns-2024-00409-Supplementary-Table-3.pdf
Supplementary Table 4. Comparison of cortical activity 3 days after treatment and before treatment in the tVNS group (correlated with Fig. 4)
kjorl-hns-2024-00409-Supplementary-Table-4.pdf
Supplementary Table 5. Comparison of cortical activity 1 month after treatment and before treatment in the tVNS group (correlated with Fig. 4)
kjorl-hns-2024-00409-Supplementary-Table-5.pdf
Supplementary Table 6. Comparison of cortical activity immediately after treatment and before treatment in the tRNS group (correlated with Fig. 4)
kjorl-hns-2024-00409-Supplementary-Table-6.pdf
Supplementary Table 7. Comparison of cortical activity 3 days after treatment and before treatment in the tRNS group (correlated with Fig. 4)
kjorl-hns-2024-00409-Supplementary-Table-7.pdf
Supplementary Table 8. Comparison of cortical activity 1 month after treatment and before treatment in the tRNS group (correlated with Fig. 4C)
kjorl-hns-2024-00409-Supplementary-Table-8.pdf
ACKNOWLEDGMENTSThis work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (NRF-2019R1H1A2039693 and 2020R1I1A3071587).
We thank librarian Hye Young Han for the manuscriptŌĆÖs technical editing and Dong Hyun Kim for his invaluable assistance in managing our laboratory.
NotesAuthor contributions Conceptualization: Eun Bit Bae, Hyun Joon Shim. Data curation: Eun Bit Bae. Formal analysis: Eun Bit Bae. Funding acquisition: Hyun Joon Shim. Investigation: Eun Bit Bae. Methodology: Eun Bit Bae, Hyun Joon Shim. Project administration: Hyun Joon Shim. Resources: Eun Bit Bae, Hyun Joon Shim. Software: Eun Bit Bae. Supervision: Hyun Joon Shim. Validation: Hyun Joon Shim. Visualization: Eun Bit Bae. WritingŌĆöoriginal draft: Eun Bit Bae, Hyun Joon Shim. WritingŌĆöreview & editing: Hyun Joon Shim. Fig.┬Ā1.Outline of investigation design. Pre, before treatment; Post-3 d, 3 days after treatment; Post-1 m, 1 month after treatment; QNR, questionnaires; EEG, electroencephalogram; S, sham treatment; R, real treatment. 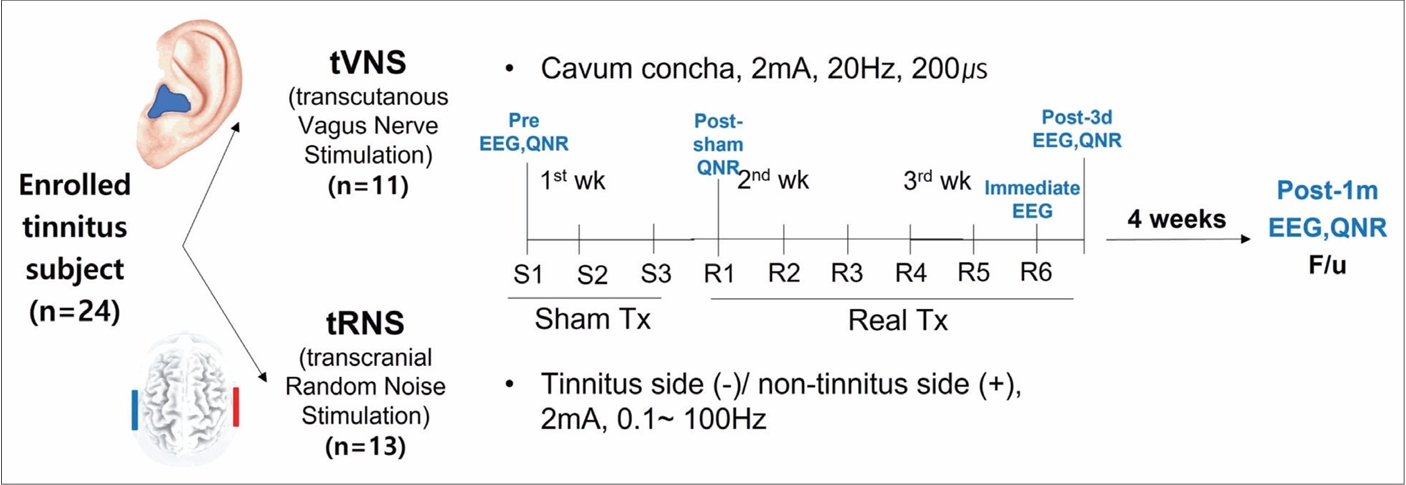
Fig.┬Ā2.CONSORT flow diagram for the randomized controlled trial of the parallel-group neuromodulation study. One subject withdrew after being randomized to the tVNS group, and three participants dropped out during treatment in the tRNS group. The final analysis excluded five participants from the tVNS group and one subject from the tRNS group, as they did not receive EEG recordings more than once at the designated time. tVNS, transcutaneous vagus nerve stimulation; tRNS, transcranial random noise stimulation; EEG, electroencephalogram. 
Fig.┬Ā3.Changes in tinnitus intensity (A), tinnitus distress (B), Tinnitus Handicap Inventory (C), and BeckŌĆÖs Depression Inventory (D) were recorded over time from before treatment to 1 month after treatment. The comparisons were performed using the Bonferroni correction. The error bar shows the standard deviation. tRNS, transcranial random noise stimulation; tVNS, transcutaneous vagus nerve stimulation; Pre, before treatment; sham, after sham treatment; Post-3 d, 3 days after treatment; Post-1 m, 1 month after treatment. *p<0.05; **p<0.01. 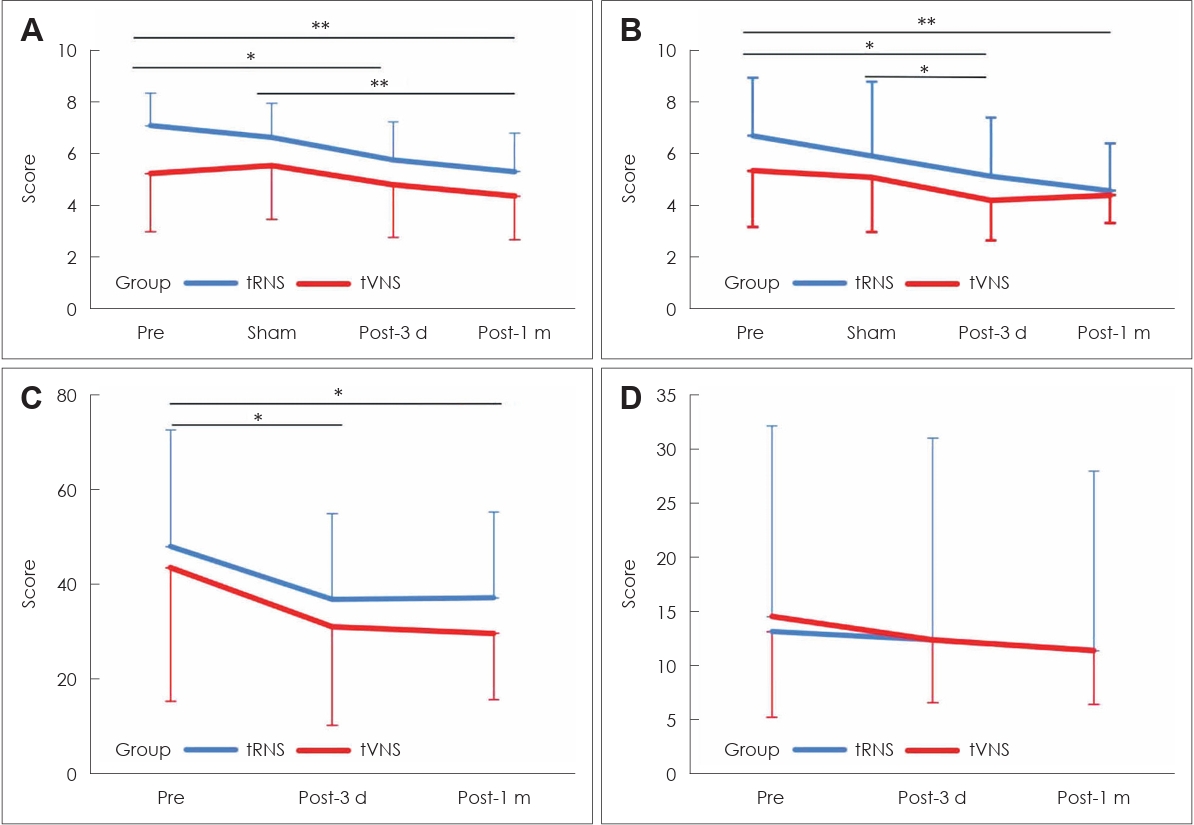
Fig.┬Ā4.Changes in neural activity were obtained by subtracting the data before treatment from the data at each time point after treatment (immediately, 3 days, and 1 month after treatment). In the scalp map, a red asterisk represents a significant difference between before treatment and each time point after treatment, as determined by a Bonferroni corrected p-value of <0.017 (A). Line graphs show the changes in the neural activity at the electrodes that exhibit a significant decrease after treatment for tVNS (B) and tRNS (C). The blue circle represents the temporal cortex electrode. tVNS, transcutaneous vagus nerve stimulation; tRNS, transcranial random noise stimulation; EEG, electroencephalogram; Pre, before treatment; Immediate, immediately after treatment; Post-3 d, 3 days after treatment; Post-1 m, 1 month after treatment. 
Fig.┬Ā5.Correlation results between changes in neural activity and subjective symptoms. A: tVNS correlation results between changes in neural activity and tinnitus distress or THI score 3 days after tVNS. B: tRNS correlation results between changes in neural activity and tinnitus distress or intensity 3 days after tRNS. Positive correlations are shown in red, and negative correlations are shown in blue in the matrix. tVNS, transcutaneous vagus nerve stimulation; tRNS, transcranial random noise stimulation; THI, Tinnitus Handicap Inventory. 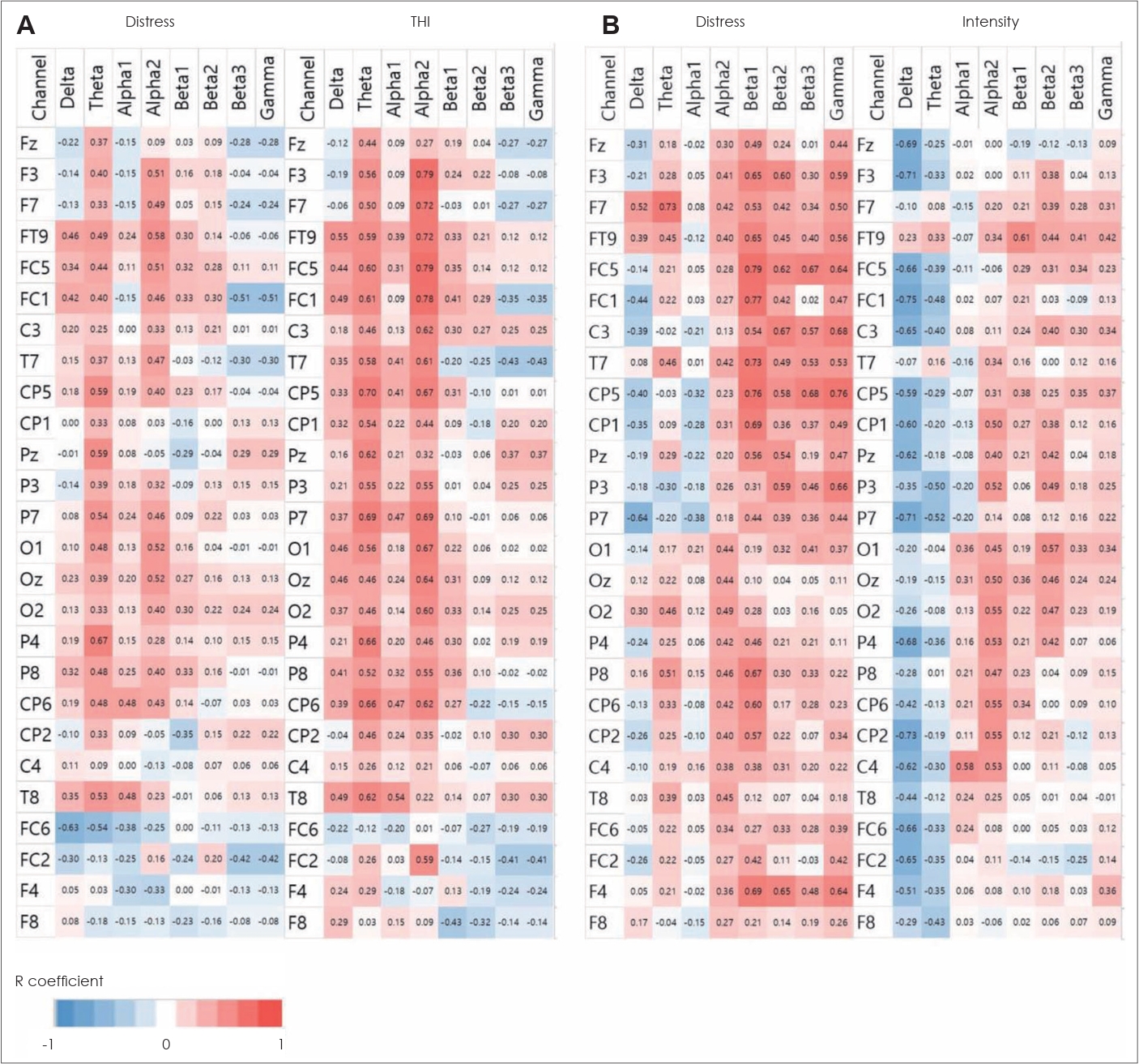
Table┬Ā1.Repeated measures analysis of variance table
Table┬Ā2.Post hoc test results using Bonferroni correction
REFERENCES1. Bartels H, Staal MJ, Albers FW. Tinnitus and neural plasticity of the brain. Otol Neurotol 2007;28(2):178-84.
2. Eggermont JJ. The auditory cortex and tinnitus ŌĆō a review of animal and human studies. Eur J Neurosci 2015;41(5):665-76.
3. Nore├▒a AJ, Farley BJ. Tinnitus-related neural activity: theories of generation, propagation, and centralization. Hear Res 2013;295:161-71.
4. Shore SE, Koehler S, Oldakowski M, Hughes LF, Syed S. Dorsal cochlear nucleus responses to somatosensory stimulation are enhanced after noise-induced hearing loss. Eur J Neurosci 2008;27(1):155-68.
5. Auerbach BD, Rodrigues PV, Salvi RJ. Central gain control in tinnitus and hyperacusis. Front Neurol 2014;5:206.
6. Parra LC, Pearlmutter BA. Illusory percepts from auditory adaptation. J Acoust Soc Am 2007;121(3):1632-41.
7. Salvi R, Sun W, Ding D, Chen GD, Lobarinas E, Wang J, et al. Inner hair cell loss disrupts hearing and cochlear function leading to sensory deprivation and enhanced central auditory gain. Front Neurosci 2017;10:621.
8. Elgoyhen AB, Langguth B, De Ridder D, Vanneste S. Tinnitus: perspectives from human neuroimaging. Nat Rev Neurosci 2015;16(10):632-42.
9. Eggermont JJ, Tass PA. Maladaptive neural synchrony in tinnitus: origin and restoration. Front Neurol 2015;6:29.
10. Shore SE, Roberts LE, Langguth B. Maladaptive plasticity in tinnitus--triggers, mechanisms and treatment. Nat Rev Neurol 2016;12(3):150-60.
11. De Ridder D, Elgoyhen AB, Romo R, Langguth B. Phantom percepts: tinnitus and pain as persisting aversive memory networks. Proc Natl Acad Sci U S A 2011;108(20):8075-80.
13. Inukai Y, Saito K, Sasaki R, Kotan S, Nakagawa M, Onishi H. Influence of transcranial direct current stimulation to the cerebellum on standing posture control. Front Hum Neurosci 2016;10:325.
14. Vanneste S, Fregni F, De Ridder D. Head-to-head comparison of transcranial random noise stimulation, transcranial AC stimulation, and transcranial DC stimulation for tinnitus. Front Psychiatry 2013;4:158.
15. Woods AJ, Antal A, Bikson M, Boggio PS, Brunoni AR, Celnik P, et al. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin Neurophysiol 2016;127(2):1031-48.
16. Rauschecker JP, Leaver AM, M├╝hlau M. Tuning out the noise: limbic-auditory interactions in tinnitus. Neuron 2010;66(6):819-26.
17. Schlee W, Schecklmann M, Lehner A, Kreuzer PM, Vielsmeier V, Poeppl TB, et al. Reduced variability of auditory alpha activity in chronic tinnitus. Neural Plast 2014;2014:436146.
18. De Ridder D, Vanneste S. EEG driven tDCS versus bifrontal tDCS for tinnitus. Front Psychiatry 2012;3:84.
19. Frank E, Schecklmann M, Landgrebe M, Burger J, Kreuzer P, Poeppl TB, et al. Treatment of chronic tinnitus with repeated sessions of prefrontal transcranial direct current stimulation: outcomes from an open-label pilot study. J Neurol 2012;259(2):327-33.
20. Shekhawat GS, Vanneste S. Optimization of transcranial direct current stimulation of dorsolateral prefrontal cortex for tinnitus: a non-linear dose-response effect. Sci Rep 2018;8(1):8311.
21. Claes L, Stamberger H, Van de Heyning P, De Ridder D, Vanneste S. Auditory cortex tACS and tRNS for tinnitus: single versus multiple sessions. Neural Plast 2014;2014:436713.
22. Joos K, De Ridder D, Vanneste S. The differential effect of low-versus high-frequency random noise stimulation in the treatment of tinnitus. Exp Brain Res 2015;233(5):1433-40.
23. Mohsen S, Mahmoudian S, Talebian S, Pourbakht A. Prefrontal and auditory tRNS in sequence for treating chronic tinnitus: a modified multisite protocol. Brain Stimul 2018;11(5):1177-9.
24. Mohsen S, Mahmoudian S, Talebian S, Pourbakht A. Multisite transcranial random noise stimulation (tRNS) modulates the distress network activity and oscillatory powers in subjects with chronic tinnitus. J Clin Neurosci 2019;67:178-84.
25. To WT, Ost J, Hart J Jr, De Ridder D, Vanneste S. The added value of auditory cortex transcranial random noise stimulation (tRNS) after bifrontal transcranial direct current stimulation (tDCS) for tinnitus. J Neural Transm (Vienna) 2017;124(1):79-88.
26. Bonaz B, Picq C, Sinniger V, Mayol JF, Claren├¦on D. Vagus nerve stimulation: from epilepsy to the cholinergic anti-inflammatory pathway. Neurogastroenterol Motil 2013;25(3):208-21.
27. Kalia M, Sullivan JM. Brainstem projections of sensory and motor components of the vagus nerve in the rat. J Comp Neurol 1982;211(3):248-65.
28. Lulic D, Ahmadian A, Baaj AA, Benbadis SR, Vale FL. Vagus nerve stimulation. Neurosurg Focus 2009;27(3):E5.
29. Rutecki P. Anatomical, physiological, and theoretical basis for the antiepileptic effect of vagus nerve stimulation. Epilepsia 1990;31(Suppl 2):S1-6.
30. Zabara J. Time course of seizure control to brief, repetitive stimuli. Epilepsia 1985;26:518.
31. Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science 1998;279(5357):1714-8.
32. Engineer ND, Riley JR, Seale JD, Vrana WA, Shetake JA, Sudanagunta SP, et al. Reversing pathological neural activity using targeted plasticity. Nature 2011;470(7332):101-4.
33. Tyler R, Cacace A, Stocking C, Tarver B, Engineer N, Martin J, et al. Vagus nerve stimulation paired with tones for the treatment of tinnitus: a prospective randomized double-blind controlled pilot study in humans. Sci Rep 2017;7(1):11960.
34. Lehtim├żki J, Hyv├żrinen P, Ylikoski M, Bergholm M, M├żkel├ż JP, Aarnisalo A, et al. Transcutaneous vagus nerve stimulation in tinnitus: a pilot study. Acta Otolaryngol 2013;133(4):378-82.
35. Shim HJ, Kwak MY, An YH, Kim DH, Kim YJ, Kim HJ. Feasibility and safety of transcutaneous vagus nerve stimulation paired with notched music therapy for the treatment of chronic tinnitus. J Audiol Otol 2015;19(3):159-67.
36. Henry JA, Roberts LE, Caspary DM, Theodoroff SM, Salvi RJ. Underlying mechanisms of tinnitus: review and clinical implications. J Am Acad Audiol 2014;25(1):5-22, quiz 126.
37. Dong C, Chen C, Wang T, Gao C, Wang Y, Guan X, et al. Low-frequency repetitive transcranial magnetic stimulation for the treatment of chronic tinnitus: a systematic review and meta-analysis of randomized controlled trials. Biomed Res Int 2020;2020:3141278.
38. Lefaucheur JP, Andr├®-Obadia N, Antal A, Ayache SS, Baeken C, Benninger DH, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin Neurophysiol 2014;125(11):2150-206.
39. Yakunina N, Nam EC. Direct and transcutaneous vagus nerve stimulation for treatment of tinnitus: a scoping review. Front Neurosci 2021;15:680590.
40. Vanneste S, Martin J, Rennaker RL 2nd, Kilgard MP. Pairing sound with vagus nerve stimulation modulates cortical synchrony and phase coherence in tinnitus: an exploratory retrospective study. Sci Rep 2017;7(1):17345.
41. Kreuzer PM, Poeppl TB, Rupprecht R, Vielsmeier V, Lehner A, Langguth B, et al. Daily high-frequency transcranial random noise stimulation of bilateral temporal cortex in chronic tinnitus - a pilot study. Sci Rep 2019;9(1):12274.
42. Kandel ER. The molecular biology of memory storage: a dialog between genes and synapses. Biosci Rep 2004;24(4-5):475-522.
43. Kandel ER, Dudai Y, Mayford MR. The molecular and systems biology of memory. Cell 2014;157(1):163-86.
44. OŌĆÖDell TJ, Kandel ER. Low-frequency stimulation erases LTP through an NMDA receptor-mediated activation of protein phosphatases. Learn Mem 1994;1(2):129-39.
45. Grover LM, Teyler TJ. Two components of long-term potentiation induced by different patterns of afferent activation. Nature 1990;347(6292):477-9.
46. Hyv├żrinen P, Yrttiaho S, Lehtim├żki J, Ilmoniemi RJ, M├żkitie A, Ylikoski J, et al. Transcutaneous vagus nerve stimulation modulates tinnitus-related beta- and gamma-band activity. Ear Hear 2015;36(3):e76-85.
47. Forogh B, Mirshaki Z, Raissi GR, Shirazi A, Mansoori K, Ahadi T. Repeated sessions of transcranial direct current stimulation for treatment of chronic subjective tinnitus: a pilot randomized controlled trial. Neurol Sci 2016;37(2):253-9.
48. Garin P, Gilain C, Van Damme JP, de Fays K, Jamart J, Ossemann M, et al. Short- and long-lasting tinnitus relief induced by transcranial direct current stimulation. J Neurol 2011;258(11):1940-8.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

 |
 |